Abstract
Purpose
To investigate the overall expression of extracellular matrix (ECM) and adhesion molecule genes using a gene array technique in the joint capsule of a frozen shoulder.
Materials and Methods
Tissues from 20 human shoulder joint capsules were harvested intraoperatively from patients (15 primary frozen shoulders, 5 controls) in our hospital. The RNA was isolated from the capsule tissue and the gene expression of ECM and adhesion molecules was analyzed using an oligo-array technique.
Results
The expression of several genes of the ECM and cell adhesion molecules was significantly higher in the capsule tissue from patients with a frozen shoulder than the controls. The gene expression of the collagen V α1/α3, VI α2/α3, VIII α1/α2, XV α1, XVIII α1 and ECM proteins including CD 44, connective tissue growth factor (CTGF), matrix metalloproteinase (MMP)-9/14, osteonectin, veriscan, hyaluronan synthase (HAS)-1, extra-cellular matrix (ECM)-1, secreted phosphoprotein (SSP)-1, tenascin C (TNC), thrombospondin 2/4 was two times higher in the frozen shoulder than the control. Several cell adhesion molecules genes including catenin α1, seletin p, integrin α3, β2, β4, β5 and laminin α4, α5 were also two times higher in the in the patients with a frozen shoulder than the control.
Figures and Tables
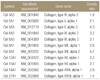
 | Figure 1Representative examples of microarray blots of human ECM and adhesion molecules from capsule tissue of the frozen shoulder patient and control. (A) Each spot on the microarray membrane represents the expression of an individual gene. For example, the red arrow indicate the MMP-9 gene on the membrane, which is expressed as dark black dot on the frozen shoulder compared with gray dot on the control shoulder. (B) The map of 128 genes on the membrane of GEArray® Series for Human Extracellular Matrix & Adhesion Molecules (Bioscience Corporation, Fredrick, MD). β-2-microglobulin (B2M) and β-actin (ACTB) gene are considered as a control genes, which should have the same expression on both membranes. |
Table 1
Collagens List of Up-regulated Genes in Capsular Specimen from the Patients with Frozen Shoulder (p<0.05)
References
1. Ramage SC, Urban NH, Jiranek WA, Maiti A, Beckman MJ. Expression of RANKL in osteolytic membranes: association with fibroblastic cell markers. J Bone Joint Surg Am. 2007. 89:841–848.
2. Rodeo SA, Hannafin JA, Tom J, Warren RF, Wickiewicz TL. Immunolocalization of cytokines and their receptors in adhesive capsulitis of the shoulder. J Orthop Res. 1997. 15:427–436.

3. Simmonds FA. Shoulder pain with particular reference to the frozen shoulder. J Bone Joint Surg Am. 1949. 31B:426–432.

4. Hannafin JA, Chiaia TA. Adhesive capsulitis. A treatment approach. Clin Orthop Relat Res. 2000. 372:95–109.
5. Border WA, Noble NA. Transforming growth factor beta in tissue fibrosis. N Engl J Med. 1994. 331:1286–1292.
6. Kilian O, Pfeil U, Wenisch S, Heiss C, Kraus R, Schnettler R. Enhanced alpha1(I) mRNA expression in frozen shoulder and dupuytren tissue. Eur J Med Res. 2007. 12:585–590.
7. Van der Zee E, Everts V, Hoeben K, Beertsen W. Cytokines modulate phagocytosis and intracellular digestion of collagen fibrils by fibroblasts in rabbit periosteal explants. Inverse effects on procollagenase production and collagen phagocytosis. J Cell Sci. 1995. 108:3307–3315.

8. Bunker TD, Anthony PP. The pathology of frozen shoulder. A Dupuytren-like disease. J Bone Joint Surg Br. 1995. 77:677–683.

9. Tauro JC. Stiffness and rotator cuff tears: incidence, arthro-scopic findings, and treatment results. Arthroscopy. 2006. 22:581–586.

10. Grey RG. The natural history of "idiopathic" frozen shoulder. J Bone Joint Surg Am. 1978. 60:564.

11. Uitvlugt G, Detrisac DA, Johnson LL, Austin MD, Johnson C. Arthroscopic observations before and after manipulation of frozen shoulder. Arthroscopy. 1993. 9:181–185.

12. Ogilvie-Harris DJ, Myerthall S. The diabetic frozen shoulder: arthroscopic release. Arthroscopy. 1997. 13:1–8.

13. Ko JY, Wang FS, Huang HY, Wang CJ, Tseng SL, Hsu C. Increased IL-1 beta expression and myofibroblast recruitment in subacromial bursa is associated with rotator cuff lesions with shouder stiffness. J Orthop Res. 2008. 26:1090–1097.
14. Meliconi R, Pulsatelli L, Melchiorri C, et al. Synovial expression of cell adhesion molecules in polymyalgia rheumatic. Clin Exp Immunol. 1997. 107:494–500.
15. Pushpakom SP, Herrick AL, Kumar S, Worthington J. Polymorphisms in COL15 gene are not associated with systemic sclerosis. J Rheumatol. 2008. 35:251–253.
16. Bunker TD, Reilly J, Baird KS, Hamblen DL. Expression of growth factors, cytokines and matrix metalloproteinases in frozen shoulder. J Bone Joint Surg Br. 2000. 82:768–773.

17. Kyriakides TR, Maclauchlan S. The role of thrombospondins in wound healing, ischemia, and the foreign body reaction. J Cell Commun Signal. 2009. 3:215–225.

18. Paddock HN, Schultz GS, Baker HV, et al. Analysis of gene expression patterns in human postburn hypertrophic scars. J Burn Care Rehabil. 2003. 24:371–377.

19. Bensadoun ES, Burke AK, Hogg JC, Roberts CR. Proteoglycans in granulomatous lung diseases. Eur Respir J. 1997. 10:2731–2737.

20. Khan SA, Cook AC, Kappil M. Enhanced cell surface CD44 variant (v6, v9) expression by osteopontin in breast cancer epithelial cells facilitates tumor cell migration: novel postranscriptional, post-translational regulation. Clin Exp Metastasis. 2005. 22:663–673.
21. Laurent TC, Fraser JR. Hyaluronan. FASEB J. 1992. 6:2397–2404.
22. Kurokawa A, Nagata M, Kitamura N, et al. Diagnostic value of integrin alpha3, beta4, and beta5 gene expression levels for the clinical outcome of tongue squamous cell carcinoma. Cancer. 2008. 112:1272–1281.
23. DeHahn KC, Gonzales M, Gonzalez AM, et al. The alpha4 laminin subunit regulates endothelial cell survival. Exp Cell Res. 2004. 294:281–289.
24. Li J, Rao H, Burkin D, Kaufman SJ, Wu C. The muscle integrin binding protein (MIBP) interacts with alpha7beta1 integrin and regulates cell adhesion and laminin matrix deposition. Dev Biol. 2003. 261:209–219.
25. Velling T, Kusche-Gullberg M, Sejersen T, Gullberg D. cDNA cloning and chromosomal localization of human alpha(11) integrin. A collagen-binding, I domain-containing, beta(1)-associated integrin alpha-chain present in muscle tissues. J Biol Chem. 1999. 274:25735–25742.
26. Blaschke UK, Eikenberry EF, Hulmes DJ, Galla HJ, Bruckner P. Collagen XI nucleates self-assembly and limits lateral growth of cartilage fibrils. J Biol Chem. 2000. 275:10370–10378.

27. Neer CS 2nd, Satterlee CC, Dalsey RM, Flatow EL. The anatomy and potential effects of contracture of the coracohumeral ligament. Clin Orthop Relat Res. 1992. 280:182–185.

28. Sattar MA, Luqman WA. Periarthritis: another duration-related complication of diabetes mellitus. Diabetes Care. 1985. 8:507–510.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download