Abstract
Purpose
To compare a processed nerve allograft, laminin derived peptide incorporated nerve conduit, and autograft in terms of electrodiagnostic testing and nerve histomorphometry for peripheral nerve regeneration in a rabbit sciatic nerve defect model.
Materials and Methods
Thirty New Zealand white rabbits were divided into three groups, and a unilateral 15 mm sciatic nerve defect was made. Group I, II and III was repaired with a reversed autograft, a processed acellular nerve allograft, and a laminin derived peptide incorporated nerve conduit, respectively. At twelve weeks, the animals were evaluated with the compound muscle action potential, wet muscle weight, and nerve histomorphometric parameters such as nerve area, number of axons, and myelin thickness.
Results
At twelve weeks, the compound muscle action potential for group I, II and III was 54.1%, 38.2% and 26.4%, respectively. Significant differences were found between the three groups (p<0.001, group I vs II; p<0.001, group I vs III; p<0.001, group II vs III). The wet muscle weight for group I, II and III was 57.8%, 54.4% and 43.9%, respectively. Group I had significantly more muscle weight than group III (p<0.001), but the difference was not significant with group II (p=0.256). Group II and III showed a significant difference (p=0.002). The number of axons in group III decreased and the shape of the axon was irregular, even though the nerve area and myelin thickness were similar in the three groups.
Figures and Tables
Figure 1
Comparison of autograft (A) and processed allograft (H & E stain, × 200). Cells are removed but intact laminin architectures are still preserved in processed allograft (B).

Figure 2
Electron micrograph of processed allograft showing preserved intact laminin structures (black arrows, ×10,000).
Figure 3
Compound muscle action potentials of the tibialis anterior for the three groups at twelve weeks. Error bars represent 95% confidence intervals. Asterisks represent significant difference between the 2 groups (p<0.05).

Figure 4
Wet muscle weight of the tibialis anterior for the three groups at twelve weeks. Error bars represent 95% confidence intervals. Asterisks represent significant difference between the 2 groups (p<0.05).

Figure 5
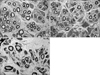
Histologic findings of the cross section of the midgraft in three groups (×4000). Autograft group (A) has more myelinated fibers than allograft (B) and conduit group (C). Regenerated nerve fibers are irregular and interstitial structures are rare in conduit group (C) than autograft group (A) and allograft group (B).
References
2. Naff NJ, Ecklund JM. History of peripheral nerve surgery techniques. Neurosurg Clin N Am. 2001. 12:197–209.

3. Meek MF, Coert JH. Clinical use of nerve conduits in peripheral-nerve repair: review of the literature. J Reconstr Microsurg. 2002. 18:97–109.

4. Staniforth P, Fisher TR. The effects of sural nerve excision in autogenous nerve grafting. Hand. 1978. 10:187–190.

5. Mackinnon SE, Dellon AL. Clinical nerve reconstruction with a bioabsorbable polyglycolic acid tube. Plast Reconstr Surg. 1990. 85:419–424.

6. Nicoli Aldini N, Perego G, Cella GD, et al. Effectiveness of a bioabsorbable conduit in the repair of peripheral nerves. Biomaterials. 1996. 17:959–962.

7. Archibald SJ, Krarup C, Shefner J, Li ST, Madison RD. A collagen-based nerve guide conduit for peripheral nerve repair: an electrophysiological study of nerve regeneration in rodents and nonhuman primates. J Comp Neurol. 1991. 306:685–696.

8. Weber RA, Breidenbach WC, Brown RE, Jabaley ME, Mass DP. A randomized prospective study of polyglycolic acid conduits for digital nerve reconstruction in humans. Plast Reconstr Surg. 2000. 106:1036–1045.

9. Yip JW, Yip YP. Laminin--developmental expression and role in axonal outgrowth in the peripheral nervous system of the chick. Brain Res Dev Brain Res. 1992. 68:23–33.
10. Mackinnon SE, Doolabh VB, Novak CB, Trulock EP. Clinical outcome following nerve allograft transplantation. Plast Reconstr Surg. 2001. 107:1419–1429.

11. Hudson TW, Zawko S, Deister C, et al. Optimized acellular nerve graft is immunologically tolerated and supports regeneration. Tissue Eng. 2004. 10:1641–1651.

12. Neubauer D, Graham JB, Muir D. Chondroitinase treatment increases the effective length of acellular nerve grafts. Exp Neurol. 2007. 207:163–170.

13. Huang YC, Huang CC, Huang YY, Chen KS. Surface modification and characterization of chitosan or PLGA membrane with laminin by chemical and oxygen plasma treatment for neural regeneration. J Biomed Mater Res A. 2007. 82:842–851.

14. de Ruiter GC, Malessy MJ, Yaszemski MJ, Windebank AJ, Spinner RJ. Designing ideal conduits for peripheral nerve repair. Neurosurg Focus. 2009. 26:E5.

15. Whitlock EL, Tuffaha SH, Luciano JP, et al. Processed allografts and type I collagen conduits for repair of peripheral nerve gaps. Muscle Nerve. 2009. 39:787–799.

16. Zuo J, Hernandez YJ, Muir D. Chondroitin sulfate proteoglycan with neurite-inhibiting activity is up-regulated following peripheral nerve injury. J Neurobiol. 1998. 34:41–54.

17. Merrell JC, Russel RC, Zook EG. Polyglycolic acid tubing as a conduit for nerve regeneration. Ann Plast Surg. 1986. 17:49–58.

18. Ide C, Tohyama K, Yokota R, Nitatori T, Onodera S. Schwann cell basal lamina and nerve regeneration. Brain Res. 1983. 288:61–75.

19. De Medinaceli L. Use of sciatic function index and walking track assessment. Microsurgery. 1990. 11:191–192.

20. Bain JR, Mackinnon SE, Hunter DA. Functional evaluation of complete sciatic, peroneal, and posterior tibial nerve lesions in the rat. Plast Reconstr Surg. 1989. 83:129–138.

21. Dellon AL, Mackinnon SE. Sciatic nerve regeneration in the rat. Validity of walking track assessment in the presence of chronic contractures. Microsurgery. 1989. 10:220–225.

22. Weber RA, Proctor WH, Warner MR, Verheyden CN. Autotomy and the sciatic functional index. Microsurgery. 1993. 14:323–327.

23. Urbanchek MS, Chung KC, Asato H, Washington LN, Kuzon WM Jr. Rat walking tracks do not reflect maximal muscle force capacity. J Reconstr Microsurg. 1999. 15:143–149.

24. Stys PK, Ransom BR, Waxman SG. Compound action potential of nerve recorded by suction electrode: a theoretical and experimental analysis. Brain Res. 1991. 546:18–32.

25. Chamberlain LJ, Yannas IV, Hsu HP, Strichartz GR, Spector M. Near-terminus axonal structure and function following rat sciatic nerve regeneration through a collagen-GAG matrix in a ten-millimeter gap. J Neurosci Res. 2000. 60:666–677.

26. Vleggeert-Lankamp CL, de Ruiter GC, Wolfs JF, et al. Pores in synthetic nerve conduits are beneficial to regeneration. J Biomed Mater Res. 2007. 80:965–982.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download