Abstract
Purpose
To evaluate the neuroprotective effect of combination therapy of polyethylene glycol (PEG) and magnesium sulfate (MgSO4) after a spinal cord injury.
Materials and Methods
Twenty Sprague Dawley male rats (300-350 gm) had a spinal cord injury after T9/10 laminectomy using an Ohio State University (OSU) impactor under intraperitoneal anesthesia. The animals were randomized to receive either PEG (1 g/kg)+MgSO4 (300 mg/kg) or saline (2 ml) via carotid vein after 2 hours of injury and then every 6 hours for 5 times. The behavioral outcome assessments were performed on days 2, 4 and 7, and then every week using the Basso, Bresnahan, and Beattie (BBB) score and subscore. The animals also underwent sensory threshold testing using a von Frey monofilament device and gait analysis with Catwalk program before and 6 weeks after cord injury. The animals were sacrificed at the end of 6 weeks and histologic assessment was performed to measure the areas of white and gray matter.
Results
For the animals treated with PEG+MgSO4 and saline, the mean BBB scores at 6 weeks post-injury were 13.3±0.3, 11.4±0.2 and the BBB subscores were 9.1±1.1, 4.4±1.2 respectively (p<0.05). No significant differences were found in sensory testing and gait analysis between the two groups. Histologic assessment revealed no significant difference in gray matter sparing but the areas of white matter at the lesion epicenter were 0.68±0.2, 0.41±0.04 mm2 in the PEG+MgSO4 and saline groups respectively, which indicated significant sparing of white matter in PEG+MgSO4 group (p<0.05).
Figures and Tables
Fig. 1
Administration of PEG+MgSO4 using port system. Injected pharmacologic agent is administrated to blood via external carotid vein.

Fig. 2
Foot prints (A-C) and gait diagram (D) using Catwalk program. (A) Forepaws only, (B) Hindpaws only, (C) All four paws combined. Regularity index, base of support, swing duration and stride length were measured.

Fig. 3
(A) BBB score. The PEG+MgSO4-treated animals showed improved open-field locomotor (BBB) scores compared with the control animals (*, p<0.05). (B) BBB subscore. The PEG+MgSO4-treated animals showed improved BBB subscores compared with the control animals (*, p<0.05).
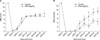
Fig. 4
Pinprick sensory test. There was no significant difference between the two groups after 6 weeks post-injury (p>0.05).
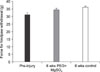
Fig. 5
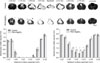
Histology assessment. There was no significant difference of grey matter sparing between the two groups. The PEG+MgSO4-treated animals showed increased sparing of the white matter at the injury epicenter and at 0.2 mm rostral and 0.2 mm caudal
(*, p<0.05).
References
1. Nobunaga AI, Go BK, Karunas RB. Recent demographic and injury trends in people served by the Model Spinal Cord Injury Care Systems. Arch Phys Med Rehabil. 1999. 80:1372–1382.

2. Celik M, Gökmen N, Erbayraktar S, et al. Erythropoietin prevents motor neuron apoptosis and neurologic disability in experimental spinal cord ischemic injury. Proc Natl Acad Sci USA. 2002. 99:2258–2263.

3. Gorio A, Gokmen N, Erbayraktar S, et al. Recombinant human erythropoietin counteracts secondary injury and markedly enhances neurological recovery from experimental spinal cord trauma. Proc Natl Acad Sci USA. 2002. 99:9450–9455.

4. Holmberg E, Nordstrom T, Gross M, Kluge B, Zhang SX, Doolen S. Simvastatin promotes neurite outgrowth in the presence of inhibitory molecules found in central nervous system injury. J Neurotrauma. 2006. 23:1366–1378.

5. Kwon BK, Tetzlaff W, Grauer JN, Beiner J, Vaccaro AR. Pathophysiology and pharmacologic treatment of acute spinal cord injury. Spine J. 2004. 4:451–464.

6. Li WW, Setzu A, Zhao C, Franklin RJ. Minocycline-mediated inhibition of microglia activation impairs oligodendrocyte progenitor cell responses and remyelination in a non-immune model of demyelination. J Neuroimmunol. 2005. 158:58–66.

7. Xu J, Kim GM, Ahmed SH, Yan P, Xu XM, Hsu CY. Glucocorticoid receptor-mediated suppression of activator protein-1 activation and matrix metalloproteinase expression after spinal cord injury. J Neurosci. 2001. 21:92–97.

8. Fournier AE, Strittmatter SM. Repulsive factors and axon regeneration in the CNS. Curr Opin Neurobiol. 2001. 11:89–94.

9. Pannu R, Barbosa E, Singh AK, Singh I. Attenuation of acute inflammatory response by atorvastatin after spinal cord injury in rats. J Neurosci Res. 2005. 79:340–350.

10. Hall ED, Springer JE. Neuroprotection and acute spinal cord injury: a reappraisal. NeuroRx. 2004. 1:80–100.

11. Bracken MB, Collins WF, Freeman DF, et al. Efficacy of methylprednisolone in acute spinal cord injury. JAMA. 1984. 251:45–52.

12. Bracken MB, Shepard MJ, Collins WF, et al. A randomized, controlled trial of methylprednisolone or naloxone in the treatment of acute spinal-cord injury. Results of the Second National Acute Spinal Cord Injury Study. N Engl J Med. 1990. 322:1405–1411.
13. Bracken MB, Shepard MJ, Holford TR, et al. Administration of methylprednisolone for 24 or 48 hours or tirilazad mesylate for 48 hours in the treatment of acute spinal cord injury. Results of the Third National Acute Spinal Cord Injury Randomized Controlled Trial. National Acute Spinal Cord Injury Study. JAMA. 1997. 277:1597–1604.

14. Wells JE, Hurlbert RJ, Fehlings MG, Yong VW. Neuroprotection by minocycline facilitates significant recovery from spinal cord injury in mice. Brain. 2003. 126:1628–1637.

15. Stepien K, Tomaszewski M, Czuczwar SJ. Neuroprotective properties of statins. Pharmacol Rep. 2005. 57:561–569.
16. Duerstock BS, Borgens RB. Three-dimensional morphometry of spinal cord injury following polyethylene glycol treatment. J Exp Biol. 2002. 205(Pt 1):13–24.

17. Borgens RB, Shi R, Bohnert D. Behavioral recovery from spinal cord injury following delayed application of polyethylene glycol. J Exp Biol. 2002. 205(Pt 1):1–12.

18. Muir KW, Lees KR. A randomized, double-blind, placebo-controlled pilot trial of intravenous magnesium sulfate in acute stroke. Stroke. 1995. 26:1183–1188.

19. Solaroglu I, Kaptanoglu E, Okutan O, Beskonakli E, Attar A, Kilinc K. Magnesium sulfate treatment decreases caspase-3 activity after experimental spinal cord injury in rats. Surg Neurol. 2005. 64:Suppl 2. 17–21.

20. Basso DM, Beattie MS, Bresnahan JC. A sensitive and reliable locomotor rating scale for open field testing in rats. J Neurotrauma. 1995. 12:1–21.

21. Popovich PG, Guan Z, Wei P, Huitinga I, van Rooijen N, Stokes BT. Depletion of hematogenous macrophages promotes partial hindlimb recovery and neuroanatomical repair after experimental spinal cord injury. Exp Neurol. 1999. 158:351–365.

22. Campbell WW. Evaluation and management of peripheral nerve injury. Clin Neurophysiol. 2008. 119:1951–1965.

23. Davies SJ, Goucher DR, Doller C, Silver J. Robust regeneration of adult sensory axons in degenerating white matter of the adult rat spinal cord. J Neurosci. 1999. 19:5810–5822.

24. Kwon BK, Fisher CG, Dvorak MF, Tetzlaff W. Strategies to promote neural repair and regeneration after spinal cord injury. Spine. 2005. 30:Suppl 17. 3–13.

25. Borgens RB, Bohnert D. Rapid recovery from spinal cord injury after subcutaneously administered polyethylene glycol. J Neurosci Res. 2001. 66:1179–1186.

26. Borgens RB, Shi R. Immediate recovery from spinal cord injury through molecular repair of nerve membranes with polyethylene glycol. FASEB J. 2000. 14:27–35.





 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download