Abstract
Purpose
We analyzed the relationship between the size of the torn rotator cuff and the frequency of finding apoptotic cells (apoptotic index) or pathological degeneration in the rotator cuff.
Materials and Methods
The edges of torn supraspinatus tendons were obtained from patients with rotator cuff tear (n=63). The study group consisted of 2 small, 22 medium, 22 large and 17 massive tears. For the histopathologic evaluation, the H&E stained sections of the torn supraspinatus tendons were examined. Apoptosis was detected with TUNEL assay. We analyzed the relationships between the tear size and the pathologic findings or the apoptotic index.
Results
Significant differences could not be found for the fibroblast cellularity, thickening of the synovial lining and proliferation of blood vessels according to the size of the rotator cuff tear. All the specimens had apoptotic cells that were concentrated around the margin of the tear site. The apoptotic indexes according to the tear size were 58.50 for the small tears, 27.25 for the medium tears, 33.29 for the large tears and 31.96 for the massive tears. No significant correlation was found between the tear size and the apoptotic index.
Conclusion
There were no significant differences in the apoptotic indices, the fibroblast cellularity, the thickening of the synovial lining and the proliferation of blood vessels according to the size of the rotator cuff tear, and there were no correlations between the apoptotic index and the histopathologic findings.
Figures and Tables
 | Fig. 1Slides from two different massive tears: Left one shows reduced fibroblast cellularity (A) and right one shows enhanced fibroblast cellularity (B) (H&E stain, ×400). |
 | Fig. 2Slides from two different massive tears: Left one shows reduced blood vessels (A) and right one shows proliferated vessels (B) (H&E stain, ×400). |
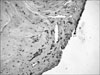 | Fig. 3A large number of dark-brown colored apoptotic cells (black arrow) were observed around the margin of the injured tendon (TUNEL stain, ×400). |
References
1. Bellumore Y, Mansat M, Assoun J. Results of the surgical repair of the rotator cuff. Radio-clinical correlation. Rev Chir Orthop Reparatrice Appar Mot. 1994. 80:582–594.
2. Galatz LM, Ball CM, Teefey SA, Middleton WD, Yamaguchi K. The outcome and repair integrity of completely arthroscopically repaired large and massive rotator cuff tears. J Bone Joint Surg Am. 2004. 86:219–224.

3. Gerber C, Fuchs B, Hodler J. The results of repair of massive tears of the rotator cuff. J Bone Joint Surg Am. 2000. 82:505–515.

4. Jost B, Pfirrmann CW, Gerber C, Switzerland Z. Clinical outcome after structural failure of rotator cuff repairs. J Bone Joint Surg Am. 2000. 82:304–314.

5. Gazielly DF, Gleyze P, Montagnon C. Functional and anatomical results after rotator cuff repair. Clin Orthop Relat Res. 1994. (304):43–53.

6. Thomazeau H, Boukobza E, Morcet N, Chaperon J, Langlais F. Prediction of rotator cuff repair results by magnetic resonance imaging. Clin Orthop Relat Res. 1997. (344):275–283.

7. Harryman DT 2nd, Mack LA, Wang KY, Jackins SE, Richardson ML, Matsen FA 3rd. Repairs of the rotator cuff. Correlation of functional results with integrity of the cuff. J Bone Joint Surg Am. 1991. 73:982–989.

8. Matthews TJ, Hand GC, Rees JL, Athanasou NA, Carr AJ. Pathology of the torn rotator cuff tendon. Reduction in potential for repair as tear size increases. J Bone Joint Surg Br. 2006. 88:489–495.
9. Nirschl RP. Rotator cuff tendinitis: basic concepts of pathoetiology. Instr Course Lect. 1989. 38:439–445.
10. Amin AR, Abramson SB. The role of nitric oxide in articular cartilage breakdown in osteoarthritis. Curr Opin Rheumatol. 1998. 10:263–268.

11. Asahara H, Hasunuma T, Obata T, Sumida T, Nishioka K. Expression of Fas/Fas ligand and proto-oncogenes in rheumatoid synovial tissues. Nippon Rinsho. 1996. 54:1960–1964.
12. Jacobson MD. Anti-apoptosis therapy: a way of treating neural degeneration? Curr Biol. 1998. 8:R418–R421.

13. Lotz M, Hashimoto S, Kuhn K. Mechanisms of chondrocyte apoptosis. Osteoarthritis Cartilage. 1999. 7:389–391.

14. Tuoheti Y, Itoi E, Pradhan RL, et al. Apoptosis in the supraspinatus tendon with stage II subacromial impingement. J Shoulder Elbow Surg. 2005. 14:535–541.

15. Yuan J, Murrell GA, Wei AQ, Wang MX. Apoptosis in rotator cuff tendonopathy. J Orthop Res. 2002. 20:1372–1379.

16. Wyllie AH. Glucocorticoid-induced thymocyte apoptosis is associated with endogenous endonuclease activation. Nature. 1980. 284:555–556.

17. Gavrieli Y, Sherman Y, Ben-Sasson SA. Identification of programmed cell death in situ via specific labeling of nuclear DNA fragmentation. J Cell Biol. 1992. 119:493–501.

18. Adams CS, Horton WE. Chondrocyte apoptosis increases with age in the articular cartilage of adult animals. Anat Rec. 1998. 250:418–425.





 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download