Abstract
Purpose
To determine the effect of methylprednisolone (MP) and riluzole administration on axonal growth after spinal cord injury (SCI) in rats.
Materials and Methods
Three Sprague Dawley rats (SD rat) served as controls (average 24 weeks of age) and 24 SCI SD rats scoring below 7 points on on Basso, Beattie, and Bresnahan open field test served as test subjects (total 27 SD rats; mean weight 581 g, range=427-613 g). Test subjects were divided into two groups of 12 subjects each. Group I was injected with saline (1 ml/kg) and group II was injected with MP (300 mg/kg) and riluzole (5 mg/kg) intraperitoneally. Four SD rats were sacrificed in each group at the following time points after SCI: days 1, 4, and 7. We completed behavioral testing, immunohistochemical staining and RT-PCR for chondroitin sulfate proteoglycans (CSPG), and microarrays for c-JUN, ATF-2, p53, and Elk-1.
Results
On behavioral testing, group II showed superior results at only day 4 after SCI (p<0.05). On RT-PCR for CSPG, optical densities were 2.06 (ratio=Group I/Group II) and 2.11 at days 4 and 7, respectively. Microarray showed that lower expression of c-JUN in group II during the entire period (p<0.05). ATF-2 showed lower expression in group II at days 4 and 7 (p<0.05). p53 showed lower expression in group I at day 1 (p<0.05). Elk-1 showed lower expression in group I at day 1 (p<0.05) and in group II at day 7 (p<0.05).
Figures and Tables
 | Fig. 1Open field score of the hind limbs after spinal cord injury in each groups. An open field score of 0 means no observable hind limb movement; an open field score 21 means normal hind limb movement. |
 | Fig. 2The immunohistochemical staining result; Noted differences of cavity size and the expression of CSPG (arrow); A (normal), B (group I) and C (group II) at 7th day. |
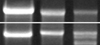 | Fig. 3This picture shows the differences of the expression of CSPG by RT-PCR in each group; more expression of CSPG in group I at 4th and 7th day. |
 | Fig. 4The results of c-JUN, ATF-2, p53 and ELK-1 by microarray: The changes of c-JUN was on the increase immediately and then drop and increment in group I, but increased with the lapse of time in group II. The degree of c-JUN in group II at 7th day was less than in group I at 1st day. The expression of ATF-2 was increased with the lapse of time but group II was less expressed than group I with statistical differences. In p53, down-regulation with the lapse of time in group I. In group II, down-regulation was noted at 4th day. In ElK-1, up-regulation in group I and II at 4th day, and then plateau in group I, but down-regulation at 7th day in group II. |
References
1. Ates O, Cayli SR, Gurses I, et al. Comparative neuroprotective effect of sodium channel blockers after experimental spinal cord injury. J Clin Neurosci. 2007. 14:658–665.

2. Baptiste DC, Fehlings MG. Pharmacological approaches to repair the injured spinal cord. J Neurotrauma. 2006. 23:318–334.

3. Basso DM, Beattie MS, Bresnahan JC. Graded histological and locomotor outcomes after spinal cord contusion using the NYU weight-drop device versus transection. Exp Neurol. 1996. 139:244–256.

4. Bracken MB, Shepard MJ, Collins WF, et al. A randomized, controlled trial of methylprednisolone or naloxone in the treatment of acute spinal-cord injury. Results of the Second National Acute Spinal Cord Injury Study. N Engl J Med. 1990. 322:1405–1411.
5. Bradbury EJ, Moon LD, Popat RJ, et al. Chondroitinase ABC promotes functional recovery after spinal cord injury. Nature. 2002. 416:636–640.

6. Deckner M, Lindholm T, Cullheim S, Risling M. Differential expression of tenascin-C, tenascin-R, tenascin/J1, and tenascin-X in spinal cord scar tissue and in the olfactory system. Exp Neurol. 2000. 166:350–362.

7. Domeniconi M, Cao Z, Spencer T, et al. Myelin-associated glycoprotein interacts with the Nogo66 receptor to inhibit neurite outgrowth. Neuron. 2002. 35:283–290.

8. Fawcett JW, Asher RA. The glial scar and central nervous system repair. Brain Res Bull. 1999. 49:377–391.

9. Frisén J, Haegerstrand A, Risling M, et al. Spinal axons in central nervous system scar tissue are closely related to laminin-immunoreactive astrocytes. Neuroscience. 1995. 65:293–304.

10. Huang CS, Song JH, Nagata K, Yeh JZ, Narahashi T. Effects of the neuroprotective agent riluzole on the high voltage-activated calcium channels of rat dorsal root ganglion neurons. J Pharmacol Exp Ther. 1997. 282:1280–1290.
11. Ikegami T, Nakamura M, Yamane J, et al. Chondroitinase ABC combined with neural stem/progenitor cell transplantation enhances graft cell migration and outgrowth of growthassociated protein-43-positive fibers after rat spinal cord injury. Eur J Neurosci. 2005. 22:3036–3046.

12. Jones LL, Margolis RU, Tuszynski MH. The chondroitin sulfate proteoglycans neurocan, brevican, phosphacan, and versican are differentially regulated following spinal cord injury. Exp Neurol. 2003. 182:399–411.

13. Jones LL, Sajed D, Tuszynski MH. Axonal regeneration through regions of chondroitin sulfate proteoglycan deposition after spinal cord injury: a balance of permissiveness and inhibition. J Neurosci. 2003. 23:9276–9288.

14. Kaneko S, Iwanami A, Nakamura M, et al. A selective Sema3A inhibitor enhances regenerative responses and functional recovery of the injured spinal cord. Nat Med. 2006. 12:1380–1389.

15. Kikuchi K, Kishino A, Konishi O, et al. In vitro and in vivo characterization of a novel semaphorin 3A inhibitor, SM-216289 or xanthofulvin. J Biol Chem. 2003. 278:42985–42991.

16. Lee HH, Yang JY, Lee JK. The impact of combined treatments with aminoguanidine and methylprednisolone on neurological recovery after spinal cord injury. J Korean Orthop Assoc. 2007. 42:444–452.

17. Lemons ML, Howland DR, Anderson DK. Chondroitin sulfate proteoglycan immunoreactivity increases following spinal cord injury and transplantation. Exp Neurol. 1999. 160:51–65.

18. Liou AK, Clark RS, Henshall DC, Yin XM, Chen J. To die or not to die for neurons in ischemia, traumatic brain injury and epilepsy: a review on the stress-activated signaling pathways and apoptotic pathways. Prog Neurobiol. 2003. 69:103–142.

19. Matsumoto T, Tamaki T, Kawakami M, Yoshida M, Ando M, Yamada H. Early complications of high-dose methylprednisolone sodium succinate treatment in the follow-up of acute cervical spinal cord injury. Spine. 2001. 26:426–430.

20. Morgenstern DA, Asher RA, Fawcett JW. Chondroitin sulphate proteoglycans in the CNS injury response. Prog Brain Res. 2002. 137:313–332.
21. Mu X, Azbill RD, Springer JE. Riluzole improves measures of oxidative stress following traumatic spinal cord injury. Brain Res. 2000. 870:66–72.

22. Nógrádi A, Szabó A, Pintér S, Vrbová G. Delayed riluzole treatment is able to rescue I njured rat spinal motoneurons. Neuroscience. 2007. 144:431–438.
23. Plant GW, Bates ML, Bunge MB. Inhibitory proteoglycan immunoreactivity is higher at the caudal than the rostral Schwann cell graft-transected spinal cord interface. Mol Cell Neurosci. 2001. 17:471–487.

25. Sun W, Gould TW, Newbern J, et al. Phosphorylation of c-Jun in avian and mammalian motoneurons in vivo during programmed cell death: an early reversible event in the apoptotic cascade. J Neurosci. 2005. 25:5595–5603.

26. Yang JY, Daniel RK. Agonist for the control of apoptosis through the study of cytokine expression after spinal cord injury in Rats. J Korean Orthop Assoc. 2007. 42:106–114.
27. Yang JY, Kim HS, Lee JK. Changes in nitric oxide synthase expression in young and adult rats after spinal cord injury. Spinal Cord. 2007. 45:731–738.





 PDF
PDF ePub
ePub Citation
Citation Print
Print





 XML Download
XML Download