Abstract
Materials and Methods
Twenty four Sprague Dawley rats had a spinal cord injury at T9/10 using an Ohio State University (OSU) impactor. The animals were randomized to receive either simvastatin, atorvastatin, or saline with oral gavage everyday for 7 days. A behavioral outcome assessment was performed on days 2, 4 and 7, and then every week using the Basso, Bresnahan, and Beattie (BBB) score and subscore. The animals also underwent sensory threshold testing using a von Frey monofilament device. The animals were sacrificed at the end of 6 weeks and a spinal cord specimen was harvested. Histology and immunohistochemistry were performed to measure the areas of white and gray matter, and the sparing of oligodenrocytes.
Results
For the animals treated with simvastatin, atorvastatin and saline, the mean BBB scores at 6 weeks post-injury was 13.2±0.1, 11.8±0.5, and 11.3±0.2 and the BBB subscores were 9.2±1.1, 4.8±1.8 and 4.4±1.4 respectively (p<0.05). The areas of white matter at the lesion epicenter were 0.78±0.05, 0.5±0.18 and 0.41±0.03 mm2 in the simvastatin, atorvastatin and saline groups respectively, and the number of spared oligodendrocytes was significantly higher in the simvastatin treated animals (p<0.05).
Figures and Tables
Fig. 1
Ohio State University (OSU) Impactor. A laminectomy (T9-10) was performed, and the bases of the adjacent spinous processes were secured with modified Allis clamps. The impactor was then triggered to deliver a 1.5 mm displacement injury at 300 m/s.

Fig. 2
(A) BBB score. The simvastatin-treated animals showed improved open-field locomotor (BBB) scores compared with the control or atorvastatin-treated animals (*p<0.05). (B) BBB subscore. The simvastatin-treated animals showed improved BBB subscores compared with the control or atorvastatin-treated animals (*p<0.05).
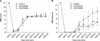
Fig. 3
Histology assessment. The simvastatin-treated animals showed increased sparing of the white matter at the injury epicenter and at 0.4 mm rostral and 0.2 mm caudal (*p<0.05).
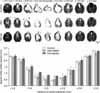
Fig. 4
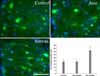
Immunohistochemistry staining. The simvastatin-treated animals showed significantly higher numbers of oligodendrocytes (as stained by Hoechst 33258 and CC1 immunohistochemistry, blue) compared with both the atorvastatin and control animals (*p<0.05).
References
1. Basso DM, Beattie MS, Bresnahan JC. A sensitive and reliable locomotor rating scale for open field testing in rats. J Neurotrauma. 1995. 12:1–21.

2. Davies SJ, Goucher DR, Doller C, Silver J. Robust regeneration of adult sensory axons in degenerating white matter of the adult rat spinal cord. J Neurosci. 1999. 19:5810–5822.

3. Di Napoli P, Taccardi AA, Oliver M, De Caterina R. Statins and stroke: evidence for cholesterol-independent effects. Eur Heart J. 2002. 23:1908–1921.

4. Fournier AE, Strittmatter SM. Repulsive factors and axon regeneration in the CNS. Curr Opin Neurobiol. 2001. 11:89–94.

5. Galandiuk S, Raque G, Appel S, Polk HC Jr. The twoedged sword of large-dose steroids for spinal cord trauma. Ann Surg. 1993. 218:419–425.

6. Hall ED, Springer JE. Neuroprotection and acute spinal cord injury: a reappraisal. NeuroRx. 2004. 1:80–100.

7. Holmberg E, Nordstrom T, Gross M, Kluge B, Zhang SX, Doolen S. Simvastatin promotes neurite outgrowth in the presence of inhibitory molecules found in central nervous system injury. J Neurotrauma. 2006. 23:1366–1378.

8. Jick H, Zornberg GL, Jick SS, Seshadri S, Drachman DA. Statins and the risk of dementia. Lancet. 2000. 356:1627–1631.

9. Kim DH, Sohn HM, Kim JJ, et al. Human umbilical cord blood infusion in paralyzed rats: histologic and behavioral alterations. J Korean Soc Spine Surg. 2007. 14:8–16.

10. Kwon BK, Borisoff JF, Tetzlaff W. Molecular targets for therapeutic intervention after spinal cord injury. Mol Interv. 2002. 2:244–258.

11. Kwon BK, Fisher CG, Dvorak MF, Tetzlaff W. Strategies to promote neural repair and regeneration after spinal cord injury. Spine. 2005. 30:Suppl 17. S3–S13.

12. Kwon BK, Oxland TR, Tetzlaff W. Animal models used in spinal cord regeneration research. Spine. 2002. 27:1504–1510.

13. Neuhaus O, Stuve O, Zamvil SS, Hartung HP. Are statins a treatment option for multiple sclerosis? Lancet Neurol. 2004. 3:369–371.

14. Nobunaga AI, Go BK, Karunas RB. Recent demographic and injury trends in people served by the Model Spinal Cord Injury Care Systems. Arch Phys Med Rehabil. 1999. 80:1372–1382.

15. Pannu R, Barbosa E, Singh AK, Singh I. Attenuation of acute inflammatory response by atorvastatin after spinal cord injury in rats. J Neurosci Res. 2005. 79:340–350.

16. Pannu R, Christie DK, Barbosa E, Singh I, Singh AK. Post-trauma Lipitor treatment prevents endothelial dysfunction, facilitates neuroprotection, and promotes locomotor recovery following spinal cord injury. J Neurochem. 2007. 101:182–200.

17. Popovich PG, Guan Z, Wei P, Huitinga I, van Rooijen N, Stokes BT. Depletion of hematogenous macrophages promotes partial hindlimb recovery and neuroanatomical repair after experimental spinal cord injury. Exp Neurol. 1999. 158:351–365.

18. Stepień K, Tomaszewski M, Czuczwar SJ. Neuroprotective properties of statins. Pharmacol Rep. 2005. 57:561–569.
19. Stirling DP, Khodarahmi K, Liu J, et al. Minocycline treatment reduces delayed oligodendrocyte death, attenuates axonal dieback, and improves functional outcome after spinal cord injury. J Neurosci. 2004. 24:2182–2190.

20. Thal SC, Engelhard K, Werner C. New cerebral protection strategies. Curr Opin Anaesthesiol. 2005. 18:490–495.

21. Vaughan CJ. Prevention of stroke and dementia with statins: effects beyond lipid lowering. Am J Cardiol. 2003. 91:23B–29B.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download