Abstract
Purpose
To evaluate the temporal and spatial expression of Transforming Growth Factor-β1 and Matrix Metalloproteinase-1 in distraction osteogenesis and fracture healing models.
Materials and Methods
Distraction osteogenesis was performed on the tibial diaphyses of Sprague-Dawley rats (latent period for 1 week, distraction for 2 weeks). The rats were euthanized at each week and the level of mRNA expression was assessed by real-time RT PCR and immunohistochemical staining.
Results
Although the level of TGF-β1 mRNA and MMP-1 mRNA expression was increased during distraction osteogenesis and fracture healing, the level of mRNA expression was significantly higher in the distraction phase in the distraction group than in the fracture healing group at the same phase. After the distraction phase, the level of mRNA expression in both groups decreased to the base line. The peak expression of mRNA was followed by that of TGF-β1 mRNA. Immunohistochemical staining revealed that TGF-β1 was expressed mainly in the osteoblast and endothelial cells, and MMP-1 was expressed mainly in the endothelial cells of the vessel.
Figures and Tables
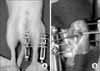 | Fig. 1Application of distraction osteogenesis. (A) Appearance of external fixator on a mouse. (B) Medial longitudinal incision and corticotomy. |
 | Fig. 2Serial examination of H&E and immunohistochemical staining in distraction osteogenesis. Seven days after the osteotomy, Hematoma organization and angiogenesis were observed. The expression of TGF-β1 and MMP-1 in the endothelial and proliferating mesenchymal cells. At 14 days, a zonal phenomenon was noted. MCF, Microcolumn formation; PMF, Primary mineralization front; FIZ, Fibrous interzone. Expression of TGF-β1 in osteoblasts in PMF was observed. At 21 days, angiogenesis was also observed in the FIZ. At 28 days, there was an increase in the amount of woven bone and extracellular matrix. |
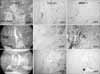 | Fig. 3Serial examination of H&E and immunohistochemical staining in the fracture healing model. At 14 days, enchondral ossification was observed. TGF-β1 expression was observed in the small proliferative chondrocytes, vessel wall and osteoblasts but not in the hypertropic chondrocytes. MMP-1 expression was observed only in the vessel wall. At 21 days, TGF-β1 expression was noted in the osteoblasts around the woven bone and vessel wall. At 28 days, the fracture gap was connected with new bone. TGF-β1 was expressed weakly on the vessel wall, and MMP-1 was expressed in the vessel wall. |
References
1. Aronson J. Temporal and spatial increases in blood flow during distraction osteogenesis. Clin Orthop Relat Res. 1994. 301:124–131.

2. Aronson J, Harrison BH, Stewart CL, Harp JH Jr. The histology of distraction osteogenesis using different external fixators. Clin Orthop Relat Res. 1989. 241:106–116.

3. Bord S, Horner A, Hembry RM, Compston JE. Stromelysin-1 (MMP-3) and stromelysin-2 (MMP-10) expression in developing human bone: potential roles in skeletal development. Bone. 1998. 23:7–12.

4. Carvalho RS, Einhorn TA, Lehmann W, et al. The role of angiogenesis in a murine tibial model of distraction osteogenesis. Bone. 2004. 34:849–861.

5. Chakraborti S, Mandal M, Das S, Mandal A, Chakraborti T. Regulation of matrix metalloproteinases: an overview. Mol Cell Biochem. 2003. 253:269–285.
6. Chang C, Werb Z. The many faces of metalloproteases: cell growth, invasion, angiogenesis and metastasis. Trends Cell Biol. 2001. 11:S37–S43.

7. Choi IH, Chung CY, Cho TJ, Yoo WJ. Angiogenesis and mineralization during distraction osteogenesis. J Korean Med Sci. 2002. 17:435–447.

8. Coassin M, Lambiase A, Micera A, Tirassa P, Aloe L, Bonini S. Nerve growth factor modulates in vitro the expression and release of TGF-beta1 by amniotic membrane. Graefes Arch Clin Exp Ophthalmol. 2006. 244:485–491.
9. Cornell CN, Lane JM. Newest factors in fracture healing. Clin Orthop Relat Res. 1992. 277:297–311.

10. Delloye C, Delefortrie G, Coutelier L, Vincent A. Bone regenerate formation in cortical bone during distraction lengthening. An experimental study. Clin Orthop Relat Res. 1990. 250:34–42.
11. Folkman J. Angiogenesis in cancer, vascular, rheumatoid and other disease. Nat Med. 1995. 1:27–31.

12. Holbein O, Neidlinger-Wilke C, Suger G, Kinzl L, Claes L. Ilizarov callus distraction produces systemic bone cell mitogens. J Orthop Res. 1995. 13:629–638.

13. Jingushi S, Scully SP, Joyce ME, Sugioka Y, Bolander ME. Transforming growth factor-beta 1 and fibroblast growth factors in rat growth plate. J Orthop Res. 1995. 13:761–768.
14. Joyce ME, Jingushi S, Bolander ME. Transforming growth factor-beta in the regulation of fracture repair. Orthop Clin North Am. 1990. 21:199–209.
15. Knabe C, Nicklin S, Yu Y, et al. Growth factor expression following clinical mandibular distraction osteogenesis in humans and its comparison with existing animal studies. J Craniomaxillofac Surg. 2005. 33:361–369.

16. Lammens J, Liu Z, Aerssens J, Dequeker J, Fabry G. Distraction bone healing versus osteotomy healing: a comparative biochemical analysis. J Bone Miner Res. 1998. 13:279–286.

17. Lewinson D, Maor G, Rozen N, Rabinovich I, Stahl S, Rachmiel A. Expression of vascular antigens by bone cells during bone regeneration in a membranous bone distraction system. Histochem Cell Biol. 2001. 116:381–388.

18. Marucci DD, Yu Y, McTavish J, Fiona Bonar S, Poole MD, Walsh WR. Matrix metalloproteinases and their inhibitors in bone remodelling following distraction osteogenesis of the sheep mandible. J Craniomaxillofac Surg. 2002. 30:208–212.

19. Mundy GR. Local control of bone formation by osteoblasts. Clin Orthop Relat Res. 1995. 313:19–26.
20. Neidlinger-Wilke C, Wilke HJ, Claes L. Cyclic stretching of human osteoblasts affects proliferation and metabolism: a new experimental method and its application. J Orthop Res. 1994. 12:70–78.

21. Nelson AR, Fingleton B, Rothenberg ML, Matrisian LM. Matrix metalloproteinases: biologic activity and clinical implications. J Clin Oncol. 2000. 18:1135–1149.

22. Robey PG, Young MF, Flanders KC, et al. Osteoblasts synthesize and respond to transforming growth factor-type beta (TGF-beta) in vitro. J Cell Biol. 1987. 105:457–463.

23. Rosen DM, Stempien SA, Thompson AY, Brennan JE, Ellingsworth LR, Seyedin SM. Differentiation of rat mesenchymal cells by cartilage-inducing factor. Enhanced phenotypic expression by dihydrocytochalasin B. Exp Cell Res. 1986. 165:127–138.
24. Rowe NM, Mehrara BJ, Luchs JS, et al. Angiogenesis during mandibular distraction osteogenesis. Ann Plast Surg. 1999. 42:470–475.

26. Sojo K, Sawaki Y, Hattori H, Mizutani H, Ueda M. Immunohistochemical study of vascular endothelial growth factor (VEGF) and bone morphogenetic protein-2, -4 (BMP-2, -4) on lengthened rat femurs. J Craniomaxillofac Surg. 2005. 33:238–245.

27. Stein GS, Lian JB. Molecular mechanisms mediating proliferation/differentiation interrelationships during progressive development of the osteoblast phenotype. Endocr Rev. 1993. 14:424–442.

28. Vu TH, Shipley JM, Bergers G, et al. MMP-9/gelatinase B is a key regulator of growth plate angiogenesis and apoptosis of hypertrophic chondrocytes. Cell. 1998. 93:411–422.

29. Weiss S, Baumgart R, Jochum M, Strasburger CJ, Bidlingmaier M. Systemic regulation of distraction osteogenesis: a cascade of biochemical factors. J Bone Miner Res. 2002. 17:1280–1289.

30. Zou S, Wang Z, Hu J. Expression of matrix metalloproteinase-3 and tissue inhibitor metalloproteinases-1 in regenerated rabbit bone after mandibular osteodistraction. Hua Xi Kou Qiang Yi Xue Za Zhi. 2003. 21:342–343. 363




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download