Abstract
Purpose
The purpose of this study was to investigate the transplantation results of human amniotic membrane (HAM), epidermal cells, or marrow mesenchymal stem cells (MSCs) in healing a skin defect.
Materials and Methods
Defects (full-thickness) in rabbits were treated with HAM alone (group A), HAM injected with cultivated epidermal cells (group B), HAM injected with cultivated MSCs (group C), or Vaseline gauze (group D). Tissue granulation, regeneration, re-epithelization and healing time were measured. Defects and healed area were calculated 2 weeks after surgery.
Results
The mean healing area was 67.5%, 81.7%, 83.2% and 49.5% in each group, with all treatment groups significantly different than group D (p<0.01), and groups B and C compared higher than group A (p<0.05). The healing time of groups A, B, and C was 5.7 to 6.4 days faster than that of group D (p<0.01). Histologic analysis showed that the new epidermis covered nearly the whole wound surface in group B and C, and contained granulated tissue with fibroblasts, capillaries, and collagen.
Figures and Tables
 | Fig. 1(A) Incision of a 3×3 cm size of full-thickness skin for collection of epidermal cells. (B) Aspiration and collection of 10 cc bone marrow mesenchymal stem cells in the rabbit femur. |
 | Fig. 2Photographs of cultivated epidermal cells and mesenchymal stem cells in vitro. (A) Epidermal cells (total cells: 6.8×106, 5 cc). (B) Mesenchymal stem cells (total cells: 6.8×106, 5 cc). |
 | Fig. 4Graft procedures in each group. (A) Sutured graft of human amniotic membrane. (B) Injection of epidermal cells or mesenchymal stem cells under the human amniotic membrane. (C) Wound covered with Vaseline gauze. |
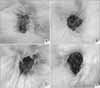 | Fig. 5Comparison of HAM alone (A), HAM with epidermal cells (B), HAM with MSCs (C), and the Vaseline gauze (D) group 1 week post-graft (HAM, human amniotic membrane; MSCs, mesenchymal stem cells). |
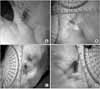 | Fig. 6Comparison of HAM alone (A), HAM with epidermal cells (B), HAM wih MSCs (C), and the vaseline gauze (D) group 2 weeks post-graft (HAM, human amniotic membrane; MSCs, mesenchymal stem cells). |
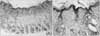 | Fig. 7Histologic finding in group A. (A) Healed tissue shows a moderately recovered, relatively thin epidermis, but a completely healed skin appendage (×40). (B) Increased vascularity, little lymphocytic infiltration, and minimal fibrosis are noted in the dermis (×200). |
 | Fig. 8Histologic finding in group B. (A) Healed tissue shows a regenerated epidermis and recovered skin appendage (×40). (B) The dermis shows a few infiltrated inflammatory cells and mild fibrosis (×200). |
 | Fig. 9Histologic finding in group C. (A) Healed tissue shows a regenerated epidermis and recovered skin appendage (×40). (B) The dermis shows mild lymphoplasma cell infiltration and hemorrhaging; with abundant vasculature and minimal fibrosis are noted (×200). |
 | Fig. 10Histologic finding in group D. (A) Healed tissue shows an irregular and flattened ridge in the epidermis and the skin appendage is mostly absent (×40). (B) The dermis shows marked fibrosis with some lymphocyte infiltration and poor vasculature (×200). |
References
1. Akle CA, Adinofi M, Welsh KI, Leibowitz S, McColl I. Immunogenicity of human amniotic epithelial cells after transplantation into volunteers. Lancet. 1981. 7:1003–1005.

2. Bennett JP, Matthews R, Faulk WP. Treatment of chronic ulceration of the legs with human amnion. Lancet. 1980. 31:1153–1156.

3. Buultmann S, You L, Spandau U. Amniotic membrane down-regulate chemokine expression in human keratocytes. Invest Ophthalmol Vis Sci. 1999. 40:3044–3060.
5. Clark RA. Cutaneous tissue repair: basic biologic consideration. J Am Acad Dermatol. 1985. 13:701–725.
6. Cunningham FG, Gant NF, Leveno KJ, et al. Williams obstetrics. 2001. 21th ed. New York: McGraw-Hill;101–105.
7. Egan TJ, O'Driscoll J, Thaker DR. Human amnion in the management of chronic ulceration of the lower limb: a clinicopathologic study. Angiology. 1983. 34:197–203.

8. Faulk WP, Matthews R, Stevens PJ, Bennett JP, Burgos H, Hsi BL. Human amnion as an adjunct in wound healing. Lancet. 1980. 31:1156–1158.

9. Jaiswal N, Haynesworth SE, Caplan AI, Bruder SP. Osteogenic differentiation of purified, culture-expanded human mesenchymal stem cells in vitro. J Cell Biochem. 1997. 64:295–312.

10. Jeung WJ, Ha SJ, Park WC, Yoo KW. The effects of amniotic membrane for prevention of adhesion in strabismus surgery in rabbits. J Korean Opthalmol Soc. 2002. 43:402–410.
11. Kim HS, Song KH, Park WC, Kim KH. Effectiveness of amniotic membrane patch in the treatment of chronic ulcers. Korean J Derma. 2005. 43:29–36.
12. Kim JC, Tseng SC. The effects on inhibition of corneal neovascularization after human amniotic membrane transplantation in severely damaged rabbit corneas. Korean J Opthalmol. 1995. 9:32–46.

13. Kim JC, Tseng SC. Transplantation of preserved human amniotic membrane for surface reconstruction in severely damaged rabbit corneas. Cornea. 1995. 14:473–484.

14. Koizumi NJ, Inatomi TJ, Sotozona CJ, Fullwood NJ, Quantock AJ, Kinoshita S. Growth factor mRNA and protein in preserved human amniotic membrane. Curr Eye Res. 2000. 20:173–177.

15. Krishnan R, Coombs R, Wright N. Coombs R, Gristina A, Hungerford D, editors. Amniotic arthroplasty - a biological alternative. Joint replacement : state of art. 1990. St Louis: Mosby;31–37.
16. Maximillian S. The grafting of preserved amniotic membrane to burned and ulcerated surfaces, substituting skin grafts. JAMA. 1913. 13:973–974.
17. Ohgushi H, Goldberg VM, Caplan AI. Repair of bone defects with marrow cells and porous ceramic. Experiments in rats. Acta Orthop Scand. 1989. 60:334–339.

18. Reed BR, Clark RA. Cutaneous tissue repair: practical implications of current knowledge. II. J Am Acad Dermatol. 1985. 13:919–941.

19. Robson MC, Krizek TJ. The effect of human amniotic membranes on the bacterial population of infected rat burns. Ann J Surg. 1973. 177:144–149.

20. Ryan JM, Barry FP, Murphy JM, Mahon BP. Mesenchymal stem cells avoid allogenic rejection. J Inflamm (Lond). 2005. 2:8.
21. Satoh H, Kishi K, Tanaka T, et al. Transplanted mesenchymal stem cells are effective for skin. regeneration in acute cutaneous wounds. Cell Transplant. 2004. 13:405–412.

22. Wakitini S, Goto T, Pineda SJ, et al. Mesenchymal cell-based repair of large, full-thickness defects of articular cartilage. J Bone Joint Surg Am. 1994. 76:579–592.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download