Abstract
Purpose
The aim of study was to compare the differentiation capacity of mesenchymal stem cells (MSCs) obtained from human bone marrow (BM) according to the age of the donors.
Materials and Methods
MSCs were isolated from the BM of young (n=16, 12.5±5.8 years) and elder (n=4, 48.5±7.2 years) patients with the consent of them. We analyzed the cell morphology and the cell surface markers of the MSCs. In addition, we assessed the cell senescence with serial cultures from both age groups. Cell pluripotentiality was analyzed by osteogenic, chondrogenic, and adipogenic induction media. We performed RT-PCR, a measurement of expression of alkaline phosphatase, and staining with von Kossa, safranin O, and oil red O stain.
Results
All of the MSC samples tested, irrespective of the age of the donors, MSCs were all successfully isolated from twenty bone marrows. However, the number of cells of from the young donors was five times greater than that of the elderly donors. Senescence was observed over 10 passages in both age groups. The immunophenotypes of both age groups showed similar patterns. MSCs obtained from young and older donors showed the potential to differentiate into osteogenic, chondrogenic, and adipogenic lineages with no difference for both age groups.
Figures and Tables
 | Fig. 1Photomicrographs showing the adherent cells obtained from human bone marrow cells. (A) Adherent cells at day 1. (B) Heterogeneous adherent cells at day 3. (C) Confluent fibroblast-like adherent cells at day 7. An homogeneous population of bipolar spindle-shaped cells was obtained. All representative examples are shown at 100×magnification. |
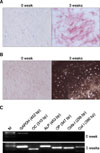 | Fig. 2Osteogenic differentiation of human BM MSCs. (A) Osteogenic induction was detected by alkaline phosphatase expression. (B) Calcium matrix mineralization was detected by the use of von Kossa stain. (C) Expression of mRNA of osteocalcin, alkaline phosphase, osteopontin, Cbfa-1 and collagen type I during osteogenic differentiation. All representative examples are shown at 100×magnification. 0 week: without osteogenic differentiation, 3 weeks: with osteogenic differentiation. |
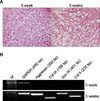 | Fig. 3Chondrogenic differentiation of human BM MSCs. (A) Proteoglycan synthesis was visualized by the use of safranin O. (B) Expression of mRNA of GAPDH, Aggrecan, Collgen type IX, Sox 9, and Collagen type II during chondrogenic differentiation. All representative examples are shown at 400×magnification. 0 week: without chondrogenic differentiation, 3 weeks: with chondrogenic differentiation. |
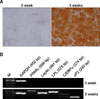 | Fig. 4Adipogenic differentiation of human BM MSCs. (A) Adipogenic induction was apparent by the accumulation of lipid vacuoles within cells, which were visualized by staining with oil red O. (B) Expression of mRNA of PPARγ, Leptin, LPL, C/EBPα, and αP2 during adipogenic differntiaiton. All representative examples are shown at 200×magnification. 0 week: without adipogenic differentiation, 3 weeks: with adipogenic differentiation. |
References
1. Colter DC, Sekiya I, Prockop DJ. Identification of a subpopulation of rapidly self renewing and multipotential adult stem cells in colonies of human stromal cells. Proc Natl Acad Sci USA. 2001. 98:7841–7845.
2. Deng W, Obrocka M, Fischer I, Prockop DJ. In vitro differentiation of human marrow stromal cells into early progenitors of neural cells by conditions that increase intracellular cyclic AMP. Biochem Biophys Res Commun. 2001. 282:148–152.

3. Digirolamo CM, Stokes D, Colter D, Phinney DG, Class R, Prockop DJ. Propagation and senescence of human marrow stromal cells in culture: a simple colony-forming assay identifies samples with the greatest potential to propagate and differentiate. Br J Haematol. 1999. 107:275–281.

4. Geiger H, Sick S, Bonifer C, Muller AM. Globin gene expressoin is reprogrammed in chimeras generated by injecting adult hematopoietic stem cells into mouse blastocysts. Cell. 1998. 93:1055–1065.
5. Horwitz EM, Gordon PL, Koo WK, et al. Isolated allogeneic bone marrow derived mesenchymal cells engraft and stimulate growth in children with osteogenesis imperfecta: Implication for cell therapy of bone. Proc Natl Acad Sci USA. 2002. 99:8932–8937.
6. Horwitz EM, Prockop DJ, Gordon PL, et al. Clinical responses to bone marrow transplantation in children with severe osteogenesis imperfecta. Blood. 2001. 97:1227–1231.

7. In't Anker PS, Noort WA, Scherjon SA, et al. Mesenchymal stem cells in human second-trimester bone marrow, liver, lung, and spleen exhibit a similar immunophenotype but a heterogeneous multilineage differentiation potential. Haematologica. 2003. 88:845–852.
8. Jaiswal N, Haynesworth SE, Caplan AI, Bruder SP. Osteogenic differentiation of purified, culture-expanded human mesenchymal stem cells in vitro. J Cell Biochem. 1997. 64:295–312.

9. Jiang Y, Jahagirdar BN, Reinhardt RL, et al. Pluripotency of mesenchymal stem cells derived from adult marrow. Nature. 2002. 418:41–49.

10. Kern S, Eichler H, Stoeve J, Kluter H, Bieback K. Comparative analysis of mesenchymal stem cells from bone marrow, umbilical cord blood, or adipose tissue. Stem Cells. 2006. 24:1294–1301.

11. Krause DS, Theise ND, Collector MI, et al. Multi-organ, multi-lineage engraftment by a single bone marrow-drived stem cell. Cell. 2001. 105:369–377.
12. Le Blanc K, Rasmusson I, Sundberg B, et al. Treatment of acute graft-versus-host disease with third party haploidentical mesenchymal stem cells. Lancet. 2004. 363:1439–1441.
13. Lee OK, Kuo TK, Chen WM, Lee KD, Hsieh SL, Chen TH. Isolation of multipotent mesenchymal stem cell from umbilical cord blood. Blood. 2004. 103:1669–1675.
14. Leskela HV, Risteli J, Niskanen S, Koivunen J, Ivaska KK, Lehenkari P. Osteoblast recruitment from stem cells does not decrease by age at late adulthood. Biochem Biophys Res Commun. 2003. 311:1008–1013.
15. Lu LL, Liu YJ, Yang SG, et al. Isolation and characterization of human umbilical cord mesenchymal stem cells with hematopoiesis-supportive function and other potentials. Haematologica. 2006. 91:1017–1026.
16. Mareschi K, Ferrero I, Rustichelli D, et al. Expansion of mesenchymal stem cells isolated from pediatric and adult donor bone marrow. J Cell Biochem. 2006. 97:744–754.

17. Minguell JJ, Erices A, Conget P. Mesenchymal stem cells. Exp Biol Med (Maywood). 2001. 226:507–520.

18. Nakamura T, Shiojima S, Hirai Y, et al. Temporal gene expression changes during adipogenesis in human mesenchymal stem cells. Biochem Biophys Res Commun. 2003. 303:306–312.

19. Pitternger MF, Mackey AM, Beck SC, et al. Multilineage potential of adult human mesenchymal stem cells. Science. 1999. 284:143–147.

20. Prockop DJ. Marrow stroma cells as stem cells for nonhematopoietic tissues. Science. 1997. 276:71–74.
21. Reyes M, Verfaillie CM. Characterization of multipotent adult progenitor cells, a subpopulation of mesenchymal stem cells. Ann N Y Acad Sci. 2001. 938:231–233.

22. Roura S, Farre J, Soler-Botija C, et al. Effect of aging on the pluripotential capacity of human CD105+ mesenchymal stem cells. Eur J Heart Fail. 2006. 8:555–563.
23. Sekiya I, Vuoristo JT, Larson BL, Prockop DJ. In vitro cartilage formation by human adult stem cells from bone marrow stroma defines the sequence of cellular and molecular events during chondrogenesis. Proc Natl Acad Sci USA. 2002. 99:4397–4402.
24. Sotiropoulou PA, Perez SA, Salaqianni M, Baxevanis CN, Papamichail M. Characterization of the optimal culture conditions for clinical scale production of human mesenchymal stem cells. Stem Cells. 2006. 24:462–471.

25. Stenderup K, Justesen J, Clausen C, Kassem M. Aging is associated with decreased maximal life span and accelerated senescence of bone marrow stromal cells. Bone. 2003. 33:919–926.

26. Wagner W, Wein F, Seckinger A, et al. Comparative characteristics of mesenchymal stem cells from human bone marrow, adipose tissue, and umbilical cord blood. Exp Hematol. 2005. 33:1402–1416.

27. Yang SE, Ha CW, Jung M, et al. Mesenchymal stem/ progenitor cells developed in cultures from UC blood. Cytotherapy. 2004. 6:476–486.




 PDF
PDF ePub
ePub Citation
Citation Print
Print






 XML Download
XML Download