Abstract
Purpose
To evaluate the effect of a combined treatment with aminoguanidine (AG) and methylprednisolone (MP) on the neurological recovery after a spinal cord injury (SCI).
Materials and Methods
SCI models (weight-drop) of Sprague Dawley rat were divided into 4 groups after the SCI. Group I was injected with normal saline, Group II with MP, Group III with AG and Group IV with MP and AG. The behavioral and immunohistochemical changes along with RT-PCR of TNFRI, TNFRII, XIAP, IL-6, and IL-6R were analyzed quantitatively and compared.
Results
At 7 days, motor recovery was observed in groups II, IV, III, and I with the level of improvement increasing in that order. Neuron cells were observed in groups II, IV=III, and I. TNFRI was not expressed in group I, but was expressed at a similar level in groups II, III and IV. TNFRII was expressed the most in group II but was not expressed in group I. The level of XIAP expression was similar to that of TNFRII. Groups II and IV showed almost no IL-6 expression, while groups I and III showed similar levels of expression. IL-6-R showed an opposite pattern to IL-6.
Figures and Tables
 | Fig. 1Open field score of the hind limbs after the spinal cord injury in each group. An open field score of 0 means no observable hind limb movement and an open field score 21 means normal hind limb movement. |
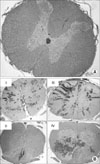 | Fig. 2(A) The findings of the normalspinal cord at 200 magnitude. (B) The histology findings of each groups at 7 days after the SCI. The number means the group. There were differences in the degree and involvement of a petechial hemorrhage in each groups. The gray matter was preserved in group II. |
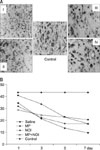 | Fig. 3(A) Nissle cresyl violet stain for each group. Note the differences in neuron cella in each group. (B) Graph showing the change in neuron cells in each group according to the time after the spinal cord injury. |
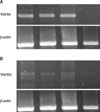 | Fig. 4(A) The results of RT-PCR for TNFRI: the order of the groups from left to right is II, III, IV and I. Note the similar pattern in groups II, III, and IV. TNFRI was not observed in group I. β-actin was used as the control. (B) RT-PCR results for TNFRII: the order of the groups from left to right is II, III, IV and I. TNFRII was expressed at different levels in groups II, III, and IV. However TNFRI was not expressed in group I. |
References
1. Basso DM, Beattie MS, Bresnahan JC. A sensitive and reliable locomotor rating scale for open field testing in rats. J Neurotrauma. 1995. 12:1–21.

2. Bracken MB. Methylprednisolone and acute spinal cord injury: an update of the randomized evidence. Spine. 2001. 26:S47–S54.
3. Carlson GD, Gorden CD, Nakazawa S, Wada E, Smith JS, LaManna JC. Sustained spinal cord compression: part II: effect of methylprednisolone on regional blood flow and recovery of somatosensory evoked potentials. J Bone Joint Surg Am. 2003. 85:95–101.
4. Fernandez E, Pallini R, Marchese E, Talamonti G. Experimental studies on spinal cord injuries in the last fifteen years. Neurol Res. 1991. 13:138–159.

5. Hulbert RJ, Moulton R. Why do you prescribe methylprednisolone for acute spinal cord injury? A Canadian perspective and a position statement. Can J Neurol Sci. 2002. 29:236–239.
6. Hurlbert RJ. Methylprednisolone for acute spinal cord injury: an inappropriate standard of care. J Neurosurg. 2000. 93:Suppl 1. S1–S7.

7. Ishikawa M, Sekizuka E, Krischek B, Sure U, Becker R, Bertalanffy H. Role of nitric oxide in the regulation of spinal arteriolar tone. Neurosurgery. 2002. 50:371–377.

8. Matsumoto T, Tamaki T, Kawakami M, Yoshida M, Ando M, Yamada H. Early complications of high-dose methylprednisolone sodium succinate treatment in the follow-up of acute cervical spinal cord injury. Spine. 2001. 26:426–430.

9. Nesathruai S. Steroids and spinal cord injury: revisiting NASCIS 2 and NASCIS 3 trials. J Trauma. 1998. 45:1088–1093.
10. Peter Vellman W, Hawkes AP, Lammertse DP. Administration of corticosteroids for acute spinal cord injury: the current practice of trauma medical directors and emergency medical system physician advisors. Spine. 2003. 28:941–947.
11. Sekhon LH, Fehlings MG. Epidemiology, demographics, and pathophysiology of acute spinal cord injury. Spine. 2001. 24:Suppl 24. S2–S12.

12. Soy O, Aslan O, Uzun H, et al. Time-level relationship for nitric oxide and the protective effects of aminoguanidine in experimental spinal cord injury. Acta Neurochir (Wien). 2004. 146:1329–1335.

14. Tator CH. Update on the pathophysiology and pathology of acute spinal cord injury. Brain Pathol. 1995. 5:407–413.

15. Yang JY, Riew KD. Agonist for the control of apoptosis through the study of cytokine expression after spinal cord injury in rats. J Korean Orthop Assoc. 2007. 42:106–114.
16. Yang JY, Lee JK, Kim YM, Kim KC, Lee CH, Kim HS. The changes of nitric oxide synthase after spinal cord injury according to the age in rats. J Korean Orthop Assoc. 2006. 41:703–710.
17. Yang JY, Lee JK, Kim KT, Lee HH, Byun BN, Ahn SH. The expression and function of the tumor necrosis factor receptor I (TNFRI), TNFRII, and X-linked inhibitor of apoptosis genes after spinal cord injury in rats. J Korean Soc Spine Surg. 2004. 11:14–23.

18. Yang JY, Lee JK, Kim SB. The changes of apoptosis according to the time of administration of methylprednisolone after spinal cord injury in rats. The Spine J. 2006. 6:5s. 23s.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download