Abstract
Purpose
To evaluate the effect of neural stem cells differentiated from a human telencephalon on the neural regeneration in the severed spinal cord.
Materials and Methods
The 1st surgery involving the insertion of plastic membrane in the transected cord was performed to prevent spontaneous healing of adult female rats (n=20, 171-237 g) with a complete spinal cord transection. The media was inserted only after removing the previously inserted plastic membrane in the control group (n=6). In the experimental group (n=14), media and neural stem cell (1×) were transplanted after removing the membrane, and immunohistochemical staining was performed. The experimental group was perfused transcardially 5 weeks after the 2nd surgery, and the level of neural cell regeneration determined by immunohistochemical staining. In behavioral analysis, the Basso-Beatie-Bresnahan (BBB) scores of the control and experimental group were compared weekly from immediately after the injury until 5 weeks post-injury after the 2nd surgery.
Results
Immunohistochemical stain revealed no neural regeneration in the control group. On the other hand, the survival of transplanted human neural stem cells and remarkable neural regeneration (differentiate to neuron and astrocyte) were observed in the experimental group. In the BBB locomotor scale, the experimental group showed significant recovery in terms of control; and the score increased from postoperative 2 weeks to 3 weeks, and reached a plateau from 3 weeks to 5 weeks.
Conclusion
The effect of neural stem cells differentiated from human telencephalon on cord regeneration does not produce functional recovery in the BBB locomotor scale, but there is slight recovery of the muscle function. The survival of transplanted human neural stem cells and the possibility of differentiation to neurons or astrocytes were observed.
Figures and Tables
Fig. 2
Photograph showing the injured spinal cord (asterix), and the insertion and removal of the plastic membrane (curved arrows) during the 1st surgery (A) and 2nd surgery (B). Showing the wide defect and surrounding scar tissue of the spinal cord result from the inserted plastic membrane.
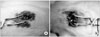
Fig. 3
Images showing β-gal (+) cell as a red color (B), Neurofilament (+) cell as a green color (A), and double staining (C) with two antibodies.

Fig. 4
Image showing β-gal (+) cell as a red color (B), GFAP (+) cell as a green color (A) and double staining (C) with two antibodies.

References
1. Alessandri G, Pagano S, Bez A, et al. Isolation and culture of human muscle-derived stem cells able to differentiate into myogenic and neurogenic cell lineages. Lancet. 2004. 364:1872–1883.

2. Ankeny DP, McTigue DM, Jakeman LB. Bone marrow transplants provide tissue protection and directional guidance for axons after contusive spinal cord injury in rats. Exp Neurol. 2004. 190:17–31.

3. Basso DM, Beattie MS, Bresnahan JC. A sensitive and reliable locomotor rating scale for open field testing in rats. J Neurotrauma. 1995. 12:1–21.

4. Blesch A, Lu P, Tuszynski MH. Neurotrophic factors, gene therapy, and neural stem cells for spinal cord repair. Brain Res Bull. 2002. 57:833–838.

5. Chu K, Kim M, Jung KH, et al. Human neural stem cell transplantation reduces spontaneous recurrent seizures following pilocarpine-induced status epilepticus in adult rats. Brain Res. 2004. 1023:213–221.

6. de Castro R Jr, Tajrishi R, Claros J, Stallcup WB. Differential responses of spinal axons to transection: influence of the NG2 proteoglycan. Exp Neurol. 2005. 192:299–309.

7. Diener PS, Bregman BS. Fetal spinal cord transplants support growth of supraspinal and segmental projections after cervical spinal cord hemisection in the neonatal rat. J Neurosci. 1998. 18:779–793.

8. Diers-Fenger M, Kirchhoff F, Kettenmann H, Levine JM, Trotter J. AN2/NG2 protein-expressing glial progenitor cells in the murine CNS: isolation, differentiation, and association with radial glia. Glia. 2001. 34:213–228.

9. Caggiano AO, Zimber MP, Ganguly A, Blight AR, Gruskin EA. Chondroitinase ABCI improves locomotion and bladder function following contusion injury of the rat spinal cord. J Neurotrauma. 2005. 22:226–239.

10. Galvin KA, Jones DG. Adult human neural stem cells for cell-replacement therapies in the central nervous system. Med J Aust. 2002. 177:316–318.

11. Hofstetter CP, Holmstrom NA, Lilja JA, et al. Allodynia limits the usefulness of intraspinal neural stem cell grafts; directed differentiation improves outcome. Nat Neurosci. 2005. 8:346–353.

12. Liu S, Qu Y, Stewart TJ, et al. Embryonic stem cells differentiate into oligodendrocytes and myelinate in culture and after spinal cord transplantation. Proc Natl Acad Sci USA. 2000. 97:6126–6131.

13. Lu P, Jones LL, Tuszynski MH. BDNF-expressing marrow stromal cells support extensive axonal growth at sites of spinal cord injury. Exp Neurol. 2005. 191:344–360.

14. Lu P, Jones LL, Snyder EY, Tuszynski MH. Neural stem cells constitutively secrete neurotrophic factors and promote extensive host axonal growth after spinal cord injury. Exp Neurol. 2003. 181:115–129.

16. Myckatyn TM, Mackinnon SE, McDonald JW. Stem cell transplantation and other novel techniques for promoting-recovery from spinal cord injury. Transpl Immunol. 2004. 12:343–358.
17. Rabchevsky AG, Fugaccia I, Turner AF, Blades DA, Mattson MP, Scheff SW. Basic fibroblast growth factor (bFGF) enhances functional recovery following severe spinal cord injury to the rat. Exp Neurol. 2000. 164:280–291.

18. Sayer FT, Oudega M, Hagg T. Neurotrophins reduce degeneration of injured ascending sensory and corticospinal motor axons in adult rat spinal cord. Exp Neurol. 2002. 175:282–296.

19. Shihabuddin LS, Horner PJ, Ray J, Gage FH. Adult spinal cord stem cells generate neurons after transplantation in the adult dentate gyrus. J Neurosci. 2000. 20:8727–8735.

20. Shihabuddin LS, Ray J, Gage FH. FGF-2 is sufficient to isolate progenitors found in the adult mammalian spinal cord. Exp Neurol. 1997. 148:577–586.

21. Yick LW, Cheung PT, So KF, Wu W. Axonal regeneration of Clarke's neurons beyond the spinal cord injury scar after treatment with chondroitinase ABC. Exp Neurol. 2003. 182:160–168.

22. Zuo J, Neubauer D, Dyess K, Ferguson TA, Muir D. Degradation of chondroitin sulfate proteoglycan enhances the neurite- promoting potential of spinal cord tissue. Exp Neurol. 1998. 154:654–662.




 PDF
PDF ePub
ePub Citation
Citation Print
Print






 XML Download
XML Download