Abstract
Purpose
To investigate the effectiveness and to compare the degree of axonal regeneration with the control after the topical application of dexamethasone, hyaluronic acid and amniotic fluid at the repair site of the peripheral nerve.
Materials and Methods
The left sciatic nerves of 40 adult Sprague-Dawley rats were transected and neurorrhaphy was performed. The nerves were divided into four groups according to the solution applied around the repair site: control group (group 1), 0.3 ml normal saline: experimental groups, 0.3 ml dexamethasone (group 2), 0.3 ml hyaluronic acid (group 3), and 0.3 ml amniotic fluid (group 4). The thickness of the perineural scar tissue, the count of the myelinated nerve fiber and axonal regeneration on the repair site were evaluated electromyographically and histologically 4 and 12 weeks after surgery.
Results
The peak amplitude of the compound motor action potential was observed in group 2, at postoperative 4 weeks and group 3 at postoperative 12 weeks. The increase in the number of myelinated nerve fibers was most prominently in group 3 at 4 and 12 weeks after surgery. The degree of perineural scarring was lowest in group 2 at postoperative 4 weeks and in group 3 at postoperative 12 weeks.
Figures and Tables
Fig. 3
The mean peak amplitude between each groups 4 and 12 weeks after surgery. The mean peak amplitude was highest in Group 2 4 weeks after surgery and 12 weeks after surgery in Group 3. The data is reported as the mean±SD.

Fig. 4
(A) There are a few myelinated nerve fibers and unmyelinated nerve fibers (black arrow) in group 1 4 weeks after surgery (TEM, ×5,000). (B) There are many myelinated nerve fibers (black arrow) 4 weeks (TEM, ×5,000) after surgery in group 3.
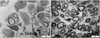
Fig. 5
(A) A few myelinated nerve fibers and unmyelinated nerve fibers were observed 12 weeks (TEM, ×5,000) after surgery in group 1. (B) Increased number of myelinated nerve fibers and unmyelinated nerve fibers is 12 weeks (TEM, ×5,000) after surgery in group 3.
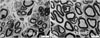
Fig. 6
The mean axonal count among each group in 4 and 12 weeks after surgery. The data is reported as the mean±SD.

Fig. 7
Longitudinal sections of the sciatic nerves taken at the repair site 4 weeks after surgery. (A) Nerve applied with saline (group 1) showing a dense band of connective scar tissue surrounding the nerve (toluidine blue, LM, ×400). (B) Nerve applied with dexamethasone (group 2) showing a thin layer of epineural scar tissue (toluidine blue, LM, ×400).

Fig. 8
Longitudinal sections of the sciatic nerves taken at the repair site 12 weeks after surgery. (A) Nerve applied with saline (group 1) showing an increase in connective scar tissue surrounding the nerve (toluidine blue, LM, ×400). (B) Nerve applied with HA (group 3) showing significantly less epineural scar tissue (toluidine blue, LM, ×400).

References
1. Becker KW, Kienecker EW, Andrae I. Effect of locally applied corticoids on the morphology of peripheral nerves following neurotmesis and microsurgical suture. Neurochirurgia (Stuttg). 1987. 30:161–167.
2. Braun S, Croizat B, Lagrange MC, Warter JM, Poindron P. Neurotrophins increase motoneurons' ability to innervate skeletal muscle fibers in rat spinal cord--human muscle cocultures. J Neurol Sci. 1996. 136:17–23.

3. Bruckner G, Delpech B, Delpech A, Girard N. Concentration of hyaluronectin and anionic glycoconjugates in perineuronal glial cell processes at GABAergic synapses of rat cerebellum. Acta Histochem. 1990. 38:Suppl. 161–165.
4. Burd DA, Greco RM, Regauer S, Longaker MT, Siebert JW, Garg HG. Hyaluronan and wound healing: a new perspective. Br J Plast Surg. 1991. 44:579–584.

5. Dahl LB, Kimpton WG, Cahill RN, Brown TJ, Fraser RE. The origin and fate of hyaluronan in amniotic fluid. J Dev Physiol. 1989. 12:209–218.
6. Da-Silva CF, Lima GM, Trezena AG. Local administration of interleukin-1 increases sensory neuron regeneration in vivo. Braz J Med Biol Res. 1990. 23:981–984.
7. Fujimoto E, Mizoguchi A, Hanada K, Yajima M, Ide C. Basic fibroblast growth factor promotes extension of regenerating axons of peripheral nerve. In vivo experiments using a Schwann cell basal lamina tube model. J Neurocytol. 1997. 26:511–528.
8. Gorgulu A, Imer M, Simsek O, Sencer A, Kutlu K, Cobanoglu S. The effect of aprotinin on extraneural scarring in peripheral nerve surgery: an experimental study. Acta Neurochir (Wien). 1998. 140:1303–1307.
9. Hagberg L. Exogenous hyaluronate as an adjunct in the prevention of adhesions after flexor tendon surgery: a controlled clinical trial. J Hand Surg Am. 1992. 17:132–136.

10. Lee HS, Kim JC. Effect of amniotic fluid in corneal sensitivity and nerve regeneration after excimer laser ablation. Cornea. 1996. 15:517–524.

11. Ljungberg C, Novikov L, Kellerth JO, Ebendal T, Wiberg M. The neurotrophins NGF and NT-3 reduce sensory neuronal loss in adult rat after peripheral nerve lesion. Neurosci Lett. 1999. 262:29–32.

12. Longaker MT, Chiu ES, Adzick NS, Stern M, Harrison MR, Stern R. Studies in fetal wound healing. V. A prolonged presence of hyaluronic acid characterizes fetal wound fluid. Ann Surg. 1991. 213:292–296.

13. Lundborg G. A 25-year perspective of peripheral nerve surgery: evolving neuroscientific concepts and clinical significance. J Hand Surg Am. 2000. 25:391–414.

14. Marx CE, Vance BJ, Jarskog LF, Chescheir NC, Gilmore JH. Nerve growth factor, brain-derived neurotrophic factor, and neurotrophin-3 levels in human amniotic fluid. Am J Obstet Gynecol. 1999. 181(5 Pt 1):1225–1230.

15. Moro-oka T, Miura H, Mawatari T, et al. Mixture of hyaluronic acid and phospholipid prevents adhesion formation on the injured flexor tendon in rabbits. J Orthop Res. 2000. 18:835–840.

16. Nath RK, Kwon B, Mackinnon SE, Jensen JN, Reznik S, Boutros S. Antibody to transforming growth factor beta reduces collagen production in injured peripheral nerve. Plast Reconstr Surg. 1998. 102:1100–1106. discussion 1107-1108.

17. Ozgenel GY. Effects of hyaluronic acid on peripheral nerve scarring and regeneration in rats. Microsurgery. 2003. 23:575–581.

18. Ozgenel GY, Filiz G. Effects of human amniotic fluid on cartilage regeneration from free perichondrial grafts in rabbits. Br J Plast Surg. 2004. 57:423–428.
19. Petersen J, Russell L, Andrus K, MacKinnon M, Silver J. Reduction of extraneural scarring by ADCON-T/N after surgical intervention. Neurosurgery. 1996. 38:976–983. discussion 983-984.

20. Rummler LS, Gupta R. Peripheral nerve repair: a review. Current Opinion in Orthopedics. 2004. 15:215–219.

21. Saika T, Senba E, Noguchi K, et al. Effects of nerve crush and transection on mRNA levels for nerve growth factor receptor in the rat facial motoneurons. Brain Res Mol Brain Res. 1991. 9:157–160.

22. Salti NI, Tuel RJ, Mass DP. Effect of hyaluronic acid on rabbit profundus flexor tendon healing in vitro. J Surg Res. 1993. 55:411–415.

23. Santos X, Rodrigo J, Hontanilla B, Bilbao G. Evaluation of peripheral nerve regeneration by nerve growth factor locally administered with a novel system. J Neurosci Methods. 1998. 85:119–127.

25. Seckel BR, Jones D, Hekimian KJ, Wang KK, Chakalis DP, Costas PD. Hyaluronic acid through a new injectable nerve guide delivery system enhances peripheral nerve regeneration in the rat. J Neurosci Res. 1995. 40:318–324.

26. Sjoberg J, Kanje M. Insulin-like growth factor I (IGF-1) as a stimulator of regeneration in the freeze-injured rat sciatic nerve. Brain Res. 1989. 485:102–108.
27. Tuncay I, Ozbek H, Atik B, Ozen S, Akpinar F. Effects of hyaluronic acid on postoperative adhesion of tendon calcaneus surgery: an experimental study in rats. J Foot Anke Surg. 2002. 41:104–108.
28. Wang K-K, Nemeth IR, Seckel BR, et al. Hyaluronic acid enhances peripheral nerve regeneration in vivo. Microsurgery. 1998. 18:270–275.

29. Wiberg M, Ljungberg C, O'Byrne A, et al. Primary sensory neuron survival following targeted administration of nerve growth factor to an injured nerve. Scand J Plast Reconstr Surg Hand Surg. 1999. 33:387–392.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download