Abstract
Purpose
The author hypothesizes that exogenously injected BMP, which is mixed with fibrin glue, can accelerate the healing of a bone-tendon junction injury and increase its holding strength during the early regeneration period.
Materials and Methods
A direct injury model of the bone-tendon junction was made using the Achilles tendon-calcaneus bone of 54 rabbits: and the transected Achilles tendon was repaired to its original insertion site using the Krackow method. In Group 1, no additional manipulation was performed. In Group 2, only fibrin glue was injected into the junction between the Achilles tendon and the calcaneus in order to exclude the effect of the fibrin glue. In Group 3, BMP-2 incorporated into the fibrin glue was injected into the junction. The results were evaluated by histological analysis and biomechanical tests at 2, 4, and 8 weeks after surgery. The Kruskal-Wallis test was used for a statistical evaluation.
Results
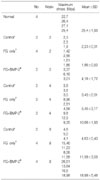
Histological analysis revealed the early appearance of fibrocartilage at 2 weeks in Group 3: the area of the fibrocartilage expanded with time. The biomechanical tests showed significant differences in the maximum stress between Groups 1 and 3, and between Groups 2 and 3, at 2, 4, and 8 weeks. 74.4% of the normal maximum stress was recovered at 8 weeks in Group 3.
Figures and Tables
 | Fig. 1Microscopic photographs of the specimen stained with H&E at 2 weeks after surgery (×100). (A) Not injected group (group I); Granulation tissues are filled in the gap of the tendon-bone junction. (B) FG injected group (group II); Granulation tissue and a small amount of fibrocartilages are filled in the tendon-bone junction. (C) BMP-2+FG injected group (group III); Granulation tissue and thick fibrocartilages (black arrow) are filled in the bone tendon junction. |
 | Fig. 2Microscopic photographs of the specimen stained with Masson-Trichrome at 2 weeks after surgery (×100). (A) Non-injected group (group I); The newly formed granulation tissues are not aligned in any direction and not organized. (B) FG injected group (group II); The newly formed granulation tissues are loosely aligned to the fibrocartilages. (C) BMP-2+FG injected group (group III); The newly formed granulation tissues are aligned at right angles to the fibrocartilages. |
 | Fig. 3Microscopic photographs of the specimen stained with H&E at 4 weeks after surgery (×100). (A) Not injected group (group I); The amount of granulation tissues is reduced and the fibrous tissues loosely directed. (B) FG injected group (group II); There is a larger amounts of fibrocartilages (black arrow) in the tendon-bone junction. (C) BMP-2+FG injected group (group III); The thickness of the fibrocartilages has increased and there is newly formed granulation tissues combined with fibrocatilages. |
 | Fig. 4Microscopic photographs of the specimen stained with Masson-Trichrome at 4 weeks after surgery (×100). (A) Non-injected group (group I); Newly formed fibrous tissues (black arrow) have direction but are loosely aligned. (B) FG injected group (group II); The amount of newly formed fibrous tissues is higher and some fibrous tissues joins the fibrocatilages (black arrow). (C) BMP-2+FG injected group (group III); The newly formed fibrous tissue are combine perpendicularly with the fibrous cartilage (black arrow). |
 | Fig. 5Microscopic images of the specimen stained with H&E at 8 weeks after surgery (×100). (A) Non-injected group (group I); Fibrocartilage tissues (black arrow) appear in the junction of the tendon and newly formed fibrous tissues fill the tendon-to bone junction. (B) FG injected group (group II); Newly formed fibrous tissue combine with the fibrocartilages and are aligned perpendicularly. (C) BMP-2+FG injected group (group III); Newly formed fibrous tissues are joined tightly to the fibrocartilage. New bone formation from the fibrocartilages can be seen at the junction and fibrocartilages stick to the bone as a result of new bone formation (black arrow). |
 | Fig. 6Microscopic images of the specimen stained with Masson-Trichrome at 4 weeks after surgery (×100). (A) Non-injected group (group I); Newly formed fibrous tissues shows good alignment and continuation. (B) FG injected group (group II); Newly formed fibrous tissues are combined tightly to the fibrous cartilages. (C) BMP-2+FG injected group (group III); Newly formed fibrous tissues are combined tightly to the fibrous cartilages perpendicularly. |
References
1. Anderson K, Seneviratne AM, Izawa K, Atkinson BL, Potter HG, Rodeo SA. Augmentation of tendon healing in an intraarticular bone tunnel with use of a bone growth factor. Am J Sports Med. 2001. 29:689–698.

2. Bosch P, Hertz H, Lintner F, Nowotny R. Does the fibrin glue accelerate the healing of tendons? (author's transl). Arch Orthop Trauma Surg. 1981. 98:305–310.
3. Ebendal T, Bengtsson H, Soderstrom S. Bone morphogenetic proteins and their receptors: potential functions in the brain. J Neurosci Res. 1998. 51:139–146.

4. Forslund C. BMP treatment for improving tendon repair. Studies on rat and rabbit Achilles tendons. Acta Orthop Scand Suppl. 2003. 74:1–30.

5. Forslund C, Aspenberg P. Improved healing of transected rabbit Achilles tendon after a single injection of cartilage-derived morphogenetic protein-2. Am J Sports Med. 2003. 31:555–559.

6. Grunder T, Gaissmaier C, Fritz J, et al. Bone morphogenetic protein (BMP)-2 enhances the expression of type II collagen and aggrecan in chondrocytes embedded in alginate beads. Osteoarthritis Cartilage. 2004. 12:559–567.
7. Itoh O. An experimental study on effect of bone morphogenetic protein and fibrin sealant in tendon implantation into bone. Nippon Seikeigeka Gakkai Zasshi. 1991. 65:580–590.
8. Jackson MR, MacPhee MJ, Drohan WN, Alving BM. Fibrin sealant: current and potential clinical applications. Blood Coagul Fibrinolysis. 1996. 7:737–746.
9. Jones CM, Dale L, Hogan BL, Wright CV, Smith JC. Bone morphogenetic protein-4 (BMP-4) acts during gastrula stages to cause ventralization of Xenopus embryos. Development. 1996. 122:1545–1554.

10. Katthagen BD. Bone induction with bone morphogenic protein. Z Orthop Ihre Grenzgeb. 1987. 125:559–566.
11. Kobayashi K, Healey RM, Sah RL, et al. Novel method for the quantitative assessment of cell migration: a study on the motility of rabbit anterior cruciate (ACL) and medial collateral ligament (MCL) cells. Tissue Eng. 2000. 6:29–38.

12. Kyung HS, Kim SY, Oh CW, Kim SJ. Tendon-to-bone tunnel healing in a rabbit model: the effect of periosteum augmentation at the tendon-to-bone interface. Knee Surg Sports Traumatol Arthrosc. 2003. 11:9–15.

13. Lattermann C, Zelle BA, Whalen JD, et al. Gene transfer to the tendon-bone insertion site. Knee Surg Sports Traumatol Arthrosc. 2004. 12:510–515.

14. Leung KS, Qin L, Fu LK, Chan CW. A comparative study of bone to bone repair and bone to tendon healing in patella-patellar tendon complex in rabbits. Clin Biomech (Bristol, Avon). 2002. 17:594–602.

15. Liu SH, Panossian V, al-Shaikh R, et al. Morphology and matrix composition during early tendon to bone healing. Clin Orthop Relat Res. 1997. 339:253–260.

16. Lusardi DA, Cain JE Jr. The effect of fibrin sealant on the strength of tendon repair of full thickness tendon lacerations in the rabbit Achilles tendon. J Foot Ankle Surg. 1994. 33:443–447.
17. Ouyang HW, Goh JC, Thambyah A, Teoh SH, Lee EH. Knitted poly-lactide-co-glycolide scaffold loaded with bone marrow stromal cells in repair and regeneration of rabbit Achilles tendon. Tissue Eng. 2003. 9:431–439.

18. Paisley PB. Observations on bone and cartilage formation in repair specimens following simple tenotomy of the rat Achilles tendon. J Anat. 1970. 106:203–204.
19. Rodeo SA, Arnoczky SP, Torzilli PA, Hidaka C, Warren RF. Tendon-healing in a bone tunnel. A biomechanical and histological study in the dog. J Bone Joint Surg Am. 1993. 75:1795–1803.

21. Schlag G, Redl H. Fibrin sealant in orthopedic surgery. Clin Orthop Relat Res. 1988. 227:269–285.

22. St Pierre P, Olson EJ, Elliott JJ, O'Hair KC, McKinney LA, Ryan J. Tendon-healing to cortical bone compared with healing to a cancellous trough. A biomechanical and histological evaluation in goats. J Bone Joint Surg Am. 1995. 77:1858–1866.

23. Thomopoulos S, Harwood FL, Silva MJ, Amiel D, Gelberman RH. Effect of several growth factors on canine flexor tendon fibroblast proliferation and collagen synthesis in vitro. J Hand Surg Am. 2005. 30:441–447.

24. Thomopoulos S, Soslowsky LJ, Flanagan CL, et al. The effect of fibrin clot on healing rat supraspinatus tendon defects. J Shoulder Elbow Surg. 2002. 11:239–247.

25. Wang CJ, Wang FS, Yang KD, et al. Shock wave therapy induces neovascularization at the tendon-bone junction. A study in rabbits. J Orthop Res. 2003. 21:984–989.

26. Ward JJ, Meyer RD, Lemons JE. Tensile strength comparison of dowel plug technique to standard techniques of tendon-bone attachment. Foot Ankle. 1988. 8:248–253.

27. Wong C, Inman E, Spaethe R, Helgerson S. Fibrin-based biomaterials to deliver human growth factors. Thromb Haemost. 2003. 89:573–582.

28. Wong MW, Qin L, Lee KM, et al. Healing of bone-tendon junction in a bone trough: a goat partial patellectomy model. Clin Orthop Relat Res. 2003. 413:291–302.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download