Abstract
Purpose
To evaluate the prevalence and pattern of perfusion defect (PD) on first-pass stress perfusion MR imaging in relation with the degree of left ventricular hypertrophy (LVH) and late gadolinium-enhancement (LGE) in patients with apical hypertrophic cardiomyopathy (APH).
Materials and Methods
Cardiac MR imaging with first-pass stress perfusion, cine, and LGE sequence was performed in 26 patients with APH from January 2008 to December 2012. We analyzed a total of 416 segments for LV wall thickness on end-diastolic phase of cine images, and evaluated the number of hypertrophied segment and number of consecutive hypertrophied segment (NCH). We assessed the presence or absence of PD and LGE from all patients. If there was PD, we subdivided the pattern into sporadic (sporadic-PD) or ring (ring-PD). Using univariate logistic method, we obtained the independent predictor for presence of overall PD and ring-PD.
Results
PD on stress perfusion MRI was observed in 20 patients (76.9%), 12 of them (60%) showed ring-PD. Maximal LV wall thickness and number of hypertrophied segment were independent predictors for overall PD (all, p < 0.05). NCH with more than 3 segments was an additional independent factor for ring-PD. However, LGE was not statistically related with PD in patients with APH.
Conclusion
About three quarters of the patients with APH showed PD, most of them represented as ring-PD. LVH degree or distribution was related with pattern of PD, however, LGE was not related with PD. Therefore, the clinical significance of PD in the patients with APH seems to be different from those with non-APH, and further comparison study between the two groups should be carried out.
Hypertrophic cardiomyopathy (HCM) is a complex and common genetic cardiac disease and it is a primary disease of the cardiac muscle that typically displays a marked and diffuse pattern of ventricular wall thickening (1). A variant apical form of HCM is characterized by wall thickening confined to the ventricular apex, with a typical 'ace of spade' configuration (2, 3). Although apical hypertrophic cardiomyopathy (APH) is generally associated with a favorable natural course, several studies have reported the occurrence of life threatening arrhythmia or significant cardiac events (4, 5).
Microvascular ischemia, which is commonly known cause of chest pain in HCM, plays an important role for development of progressive ventricular dysfunction or congestive heart failure (6, 7). Usually, the severity of perfusion impairment in HCM has been correlated with the degree of left ventricular hypertrophy (LVH) (8). These perfusion abnormalities were more frequently noted in APH than the other types of HCM. However, there is a paucity report regarding incidence of perfusion abnormality and relationship with fibrosis in APH.
For the evaluation of myocardial perfusion, nuclear imaging such as single photon emission computed tomography (SPECT) or positron emission tomography (PET) has been widely used in clinical practice. However, it is limited to detect the subendocardial perfusion defect (PD) due to poor spatial resolution and variable motion artifacts hampered the diagnostic accuracy (9). Currently, cardiac magnetic resonance imaging (CMR) has been widely adopted for perfusion stress testing, because it has high spatial resolution and the ability to quantify the PD. In addition, the ventricular apex is sometimes difficult to image by echocardiography (10), CMR is considered as appropriate tool for the evaluation of comprehensive information such as LV function and myocardial fibrosis in APH (11). Therefore, we evaluate the prevalence and pattern of PD on first-pass stress perfusion MR imaging in relation with the degree of LVH and late gadolinium-enhancement (LGE) in patients with APH.
The institutional review board approved the study protocol and informed consent was waived. We retrospectively enrolled 26 adults with APH (21 men; mean age ± SD: 57.8 ± 9.8 years; 43-78 years) who underwent CMR from January 2008 to December 2012. The diagnosis of APH was based on typical electrocardiographic (ECG) finding and echocardiographic imaging features. APH was defined as a diffuse or segmental LVH at apical wall with a non-dilated and hyper-dynamic chamber in the absence of another cardiac or systemic disease capable of producing the magnitude of hypertrophy that is evident (1). All patients had giant negative T-waves in the precordial ECG, and a characteristic 'ace of spades' configuration of the LV on echocardiography.
We excluded the obstructive coronary artery disease, atrial fibrillation or left bundle branch block on ECG, other cause of LVH including valvular heart disease or infiltrative cardiomyopathy, and systolic dysfunction with less than 50% of LV ejection fraction (EF).
We assessed basic demographic data and clinical risk factors from all patients. Clinical symptoms of patient were assessed by physician's interview. When the patients had chest pain, it is subdivided into atypical chest pain or typical angina pain on the basis of Canadian Cardiac Society angina classification (12).
Body weight, height, and blood pressure were also measured when they underwent MR scan. Body mass index was calculated by dividing the individual's body weight by the square of their height. Hypertension was defined as a systolic/diastolic blood pressure at 140/90 mmHg or higher and/or use of antihypertensive medication. Diabetes was defined by a fasting blood glucose level of 126 mg/dl or higher, or current anti-diabetic treatment. Hypercholesterolemia was defined by a total cholesterol level of 240 mg/dL or higher, or treatment for hypercholesterolemia. If patients currently smoked for at least 1 year, they were classified as current smokers. Family history of sudden cardiac death or known HCM was obtained by physician's interview.
MR studies were performed by a 1.5-T cardiac MRI unit (Intera CV release 10, Philips Healthcare) with five-element phased-array cardiac coil. ECG gating and triggering were performed using the vector cardiographic method. All patients performed stress-perfusion, cine and LGE MR imaging.
After the acquisition of survey images in the standard views, adenosine was infused at a dose of 0.14 mg/kg/min for up to 6 minutes. During the adenosine infusion, ECG activity was continuously monitored, and blood pressure and heart rate were checked every minute. The adenosine stress MR perfusion scanning was performed on last minutes of adenosine infusion, and it represented the three short-axis geometries using 40 dynamic acquisitions. During the inspiratory phase of the second breath, a bolus of gadodiamide (Omniscan, GE Healthcare) was injected using a power injector (Spectris, Medrad) into an antecubital vein at a dose of 0.1 mmol/kg of body weight and an injection rate of 4 mL/s, followed by a 20-mL saline flush. Stress perfusion MR images were obtained with a gradient-echo sequence by using saturation-recovery steady-state free precession (SR-SSFP) (TR/TE, 2,744/1,372; flip angle, 50°; matrix, 256 × 256; section thickness, 8-10 mm; intersection gap,10-20 mm). After 15-25 minutes, an identical MR perfusion scan at rest was continued to allow adequate clearance of the first bolus of the contrast agent. We also acquired 8 to 10 contiguous short-axis slices of heart to cover the entire LV and acquired images in the 2-chamber and 4-chamber views for cine images using a segmented SSFP (repetition time msec/echo time msec, 3/1.5, flip angle α60°). LGE was additionally performed using an inversion recovery fast gradient echocardiographic pulse sequence (repetition time msec/echo time msec, 4.6/1.5, flip angle α15°, inversion recovery time 200 to 280 msec).
Myocardial segments were evaluated according to the 17-segment model recommended by the American Heart Association (13). We analyzed the 16 segments at the representative short-axis slices of the basal (segments 1-6), mid-ventricular (segments 7-12), and apical (segments 13-16) regions of the LV, except for apex (segment 17). Therefore, a total of 416 segments were analyzed for 26 patients. Two experienced radiologists evaluated MR images without clinical information or result from other studies. If there was discordant region, two radiologists were finally interpreted with consensus. Image analysis was performed using an independent workstation with dedicated software (Extended MR Workspace 2.6, Philips Healthcare).
Using cine images with SSFP, maximal LV wall thickness was measured on each segment of LV at end diastolic phase. The LVH was defined as more than 15 mm on end-diastolic stage (1). Number of hypertrophied segment was defined as the total number of LVH segments. In addition, if the hypertrophied segments were noted at least two adjacent segments, we determined it as number of consecutive hypertrophied segment (NCH)-2. If the LVH was noted at least 3 adjacent segments, we classified it as NCH-3.
For assessing PD, rest and stress perfusion MR images were displayed side by side on a workstation and were evaluated by manual paging the images for differentiation of low enhancement caused by micro-vascular ischemia from artifact. On MR images, a PD was defined as a clearly visible hypoenhancement in at least one slice that persisted for at least three consecutive temporal images of the first-pass stress perfusion MRI (14, 15). According to presence or pattern, we classified the patient with APH into 1) no PD, 2) Presence of PD with sporadic pattern; PD was randomly located, and 3) ring of perfusion defect (ring-PD); ring like subendocardial hypoattenuation at apical layer. LGE was determined using 6 standard deviations (SDs) above the mean signal intensity for the normal nulled myocardium (16). Normal myocardium was defined as a region of myocardium without any apparent LGE at visual inspection. The mean signal intensity and SD were determined by drawing a region of interest in a portion of the normal myocardium.
Each categorical variable was expressed as number and percentage of patients. Continuous data was reported as the mean values ± SD. Inter-reader agreement for detection or image measurement of LV wall thickness, PD or LGE on CMR was assessed by using weighted κ statistics and interpreted as follows: poor (< 0.20), fair (0.20-0.40), moderate (0.41-0.60), good (0.61-0.80), and excellent agreement (0.81-1.00). For comparison of demographic and imaging features among three PD groups, one way ANOVA analysis was acquired. For independent predictor of the overall PD and ring-PD, we performed univariate logistic regression models. A p-value < 0.05 was considered to indicate statistical significance.
Demographics and clinical risk factors in patients with APH were shown in Table 1. The prevalence of male was 80.8% and mean age of the subjects was 57.8 ± 9.8 years. Family history of sudden cardiac death or known HCM was 3 patients (11.5%). Fifteen patients (57.7%) complained of symptoms as follows; chest pain (n=12), dyspnea (n=5), palpitation (n=3) and syncope (n=2). Among them, 7 patients had mixed symptoms such as chest pain and dyspnea, palpitation or syncope. Among the patients with chest pain, 5 patients were atypical and 7 patients were complained typical angina. Eleven patients (42.3%) were asymptomatic, but they performed MRI for risk stratification. The mean EF was 66.8 ± 6.6%, LV mass was 199.5 ± 60.9 g.
The mean maximal LV wall thickness was 18.7 ± 3.3 mm (range; 12-24 mm) in 26 patients with APH. Number of hypertrophied segment (hypertrophied segments more than 15 mm in thickness) in each patient was 4.2 ± 3.2 (range; 1-13) segments. Eighteen patients (69.2%) showed LGE on delayed enhancement MRI, these LGE were presented as multifocal patchy enhancement in hypertrophied segment of all patients.
Among 26 patients with APH, 20 patients (76.9%) showed PD and 12 of them (60.0%) represented as ring of subendocardial PD. All PD was reversible pattern, which was defined as nearly normal perfusion on rest-perfusion MR images from hypoattenuated PD on stress-perfusion MR images.
Table 2 was summarized as comparison data of demographic and imaging findings according to three groups which were classified by the presence or pattern of PD. There was no statistically significant difference among the three groups in terms of demographic data including presence of symptom. However, typical angina pain including syncope was frequently noted in ring-PD group, although it was not statistically different (p=0.152). Maximal LV wall thickness and number of hypertrophied segment were statistically different among the three groups (all, p < 0.05), indicating the maximal LV wall thickness and number of hypertrophied segment were linearly related with PD and ring-PD group.
For evaluation of the characteristics of ring-PD in comparison to no-PD group, we compared the two groups after exclusion of the sporadic-PD group. Besides LV wall thickness and number of hypertrophied segment, NCH-3 was significantly related with ring-PD (p < 0.05) (Fig. 1), although NCH-2 was not statistically associated with ring-PD (Fig. 2).
The inter-reader agreements for EF (k=0.86), LV mass (k=0.82) and LV wall thickness (k=0.85) were excellent. The agreement with the presence of overall PD and classification of PD pattern were good (k=0.72). The overall agreement of the presence of LGE was excellent (k=0.83).
The independent predictors of overall PD and ring-PD in patients with APH were summarized in Table 3. Using univariate logistic analysis, maximal LV wall thickness (odds ratio [OR], 1.97; 95% confidence interval [CI], 1.05 to 2.43; p=0.029) and number of hypertrophied segment (OR, 1.82; 95% CI, 1.01 to 3.30; p=0.048) were independent predictor for overall PD. The independent predictors for ring-PD were the maximal LV wall thickness (OR, 1.50; 95% CI, 1.06 to 2.13; p=0.021) and NCH-3 (OR, 9.00; 95% CI, 1.39 to 58.44; p=0.021). It means NCH with more than 3 segments is statistically associated with ring-PD. However, the presence of LGE was not associated with overall PD or ring-PD.
The main findings of the present study of first-pass stress perfusion MR images in patients with APH are the following; 1) PDs were shown in 76.9% of APH, 60% of PD presented as ring of subendocardial PD. 2) LV wall thickness and number of hypertrophied segment were independent factors for overall PD, and more than three consecutive hypertrophied segment was additional independent factor for ring-PD in APH. However, the presence of LGE was not statistically related with PD or ring-PD in APH.
APH is a common type of HCM in East Asia and it has known to have a relatively favorable prognosis in terms of cardiovascular mortality (17). Although the prevalence of APH has been rare in western countries, its outcome appears to be less benign than in Japan, with atrial fibrillation and myocardial infarction the most frequent complication occurring in up to a third of patients during long term follow up (18).
In APH, severe apical hypertrophy could impose pressure overload and impair coronary flow in the LV apex, thereby inducing myocardial ischemia (19). Microvascular dysfunction and structural changes including small intramural coronary arteriole dysplasia with collagen deposition in hypertrophied myocardium has been thought to be a cause of reduced coronary flow reserve in patients with HCM (5). Using several animal studies, a decreased capillary density has been measured in hypertrophied myocardium (20). These findings have led to the clinically silent myocardial ischemia, myocyte death, and replacement scars, which are cause for tachyarrhythmia (21). Many authors reported that reversible perfusion abnormalities are common in HCM (22, 23). According to these reports, about 40% of HCM shows 201 thallium defect despite normal epicardial coronary arteries (22) and about 80% of HCM shows reversible 201 thallium abnormalities during exercise stress and most likely identify myocardial ischemia (23). Lee et al. (24) reported 50% of HCM showed PD, which were predominantly reversible and mostly apical type (69.2%). Our study also showed similar results that 76.9% of APH had PD and most common pattern of PD was ring-PD. The ring of reversible PD during stress-perfusion imaging is also noted as characteristic finding of cardiac syndrome X which is characterized by angina-like chest pain and a positive exercise test, in presence of angiographically smooth coronary arteries (25). Although multiple pathogenic mechanisms have been hypothesized, it was mainly caused by coronary microvascular dysfunction (26). It was similar of reversible PD in APH caused by microvascular ischemia, although the mechanism of microvascular dysfunction is different from cardiac syndrome X. A study by Yamada et al. (27) found reversible PD did not correlate with symptom, but was associated with greater maximal LV wall thickness. These results were similar with our results of that the independent predictors for PD were maximal LV wall thickness and number of LVH regardless of symptom. We additionally found the independent factor for ring of PD was at least three adjacent LVH. Although it was not statistically significant, typical angina pain including syncope occurred in most of patients with ring-PD.
Until now, there was a controversy about whether PD in APH leads to adverse prognosis. Dilsizian et al. (28) reported the inducible ischemia detected by thallium-201 scan is frequently related to cardiac arrest and syncope in young patients with HCM, however, Lee et al. (24) reported that reversible thallium defect in the absence of coronary artery disease shows benign prognosis despite the presence of ischemia. Usually, a lot of studies reported that LGE in HCM was correlated with adverse prognosis such as sudden cardiac death, ventricular tachycardia, or heart failure symptom (29). Pathologically, LGE may show areas where replacement fibrosis has occurred following microvascular ischemia and focal necrosis (30). Therefore, PD is presented by earlier structural change of HCM, chronic impairment of myocardial perfusion and repeated inducible ischemia may lead to fibrosis. In addition, PD in APH seems to be different effect for clinical prognosis from the non-APH. Therefore, further comparison study regarding clinical outcomes of PD between APH and non-APH should be carried out.
Our current study has several limitations. First, we assess the PD and LGE by just visual assessment, not quantification. Although the presence of PD or LGE was assessed by two observers with final consensus, the severity of PD or amount of LGE was not graded. Thus, further quantitative study will be necessary for the exact correlation between PD and LGE. Second, our study did not include follow-up data. As yet there is disagreement regarding the clinical significance of PD, long-term follow-up should be carried out for obtaining more information on this aspect.
In conclusion, about three quarters of the patients with APH showed PD, most of them represented as ring-PD. LVH degree or distribution was related with pattern of PD, however, LGE was not related with PD. Therefore, the clinical significance of PD in the patients with APH seems to be different from those with non-APH, and further comparison study between the two groups should be carried out.
Figures and Tables
Fig. 1
A 45-year-old male with angina pain who visited to emergency room was confirmed as apical HCM by typical ECG and echocardiographic findings, and he performed CMR for risk stratification.
a. Two-chamber cine image shows apical wall thickening with typical 'spade of ace' sign (arrows).
b. Short-axis cine image shows LVH at apical anterior, lateral and inferior wall (NCH-3).
c, d. First-pass stress perfusion (c) and rest perfusion (d) show reversible ring of subendocardial perfusion defect (arrows) at whole
apical layer.
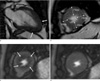
Fig. 2
A 54-year-old female with dyspnea who confirmed as apical HCM by typical ECG and echocardiographic findings.
a. Short-axis cine image shows LVH at apical anterior and septal wall (NCH-2).
b. First-pass stress perfusion show sporadic patterned subendocardial perfusion defect at apical septal wall (arrows).
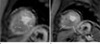
Table 2
Comparison of Demographic and Imaging Findings According to Presence or Pattern of Perfusion Defect

Note.-PD = perfusion defect, LGE = late gadolinium-enhancement, NHS = Number of hypertrophied segment, NCH-2 = more than 2 consecutive hypertrophied segments, NCH-3 = more than 3 consecutive hypertrophied segments
*p < 0.05 by one-way ANOVA analysis for comparison among the three groups
†p < 0.05 for comparison between no PD group and ring-PD group, except for sporadic-PD group
Acknowledgements
This work was supported by the grant 11-2008-004 from the Seoul National University Bundang Hospital (SNUBH) Research Fund.
References
1. Maron BJ, McKenna WJ, Danielson GK, et al. American college of cardiology/european society of cardiology clinical expert consensus document on hypertrophic cardiomyopathy. A report of the american college of cardiology foundation task force on clinical expert consensus documents and the european society of cardiology committee for practice guidelines. J Am Coll Cardiol. 2003; 42:1687–1713.
2. Sakamoto T, Tei C, Murayama M, Ichiyasu H, Hada Y. Giant T wave inversion as a manifestation of asymmetrical apical hypertrophy (AAH) of the left ventricle. Echocardiographic and ultrasono-cardiotomographic study. Jpn Heart J. 1976; 17:611–629.
3. Chun EJ, Choi SI, Jin KN, et al. Hypertrophic cardiomyopathy: Assessment with MR imaging and multidetector CT. Radiographics. 2010; 30:1309–1328.
4. Okishige K, Sasano T, Yano K, Azegami K, Suzuki K, Itoh K. Serious arrhythmias in patients with apical hypertrophic cardiomyopathy. Intern Med. 2001; 40:396–402.
5. Kusukawa J, Suwa M, Nakayama Y, et al. Advanced sequelae of apical hypertrophic cardiomyopathy: report of two cases with wall motion abnormalities. J Cardiol. 1988; 18:259–269.
6. Nishimura RA, Holmes DR Jr. Clinical practice. Hypertrophic obstructive cardiomyopathy. N Engl J Med. 2004; 350:1320–1327.
7. Spirito P, Bellone P. Natural history of hypertrophic cardiomyopathy. Br Heart J. 1994; 72:6 Suppl. S10–S12.
8. Sipola P, Lauerma K, Husso-Saastamoinen M, et al. First-pass MR imaging in the assessment of perfusion impairment in patients with hypertrophic cardiomyopathy and the Asp175Asn mutation of the alpha-tropomyosin gene. Radiology. 2003; 226:129–137.
9. Salerno M, Beller GA. Noninvasive assessment of myocardial perfusion. Circ Cardiovasc Imaging. 2009; 2:412–424.
10. Moon JC, Fisher NG, McKenna WJ, Pennell DJ. Detection of apical hypertrophic cardiomyopathy by cardiovascular magnetic resonance in patients with non-diagnostic echocardiography. Heart. 2004; 90:645–649.
11. Writing Committee Members. Yancy CW, Jessup M, et al. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation. 2013; 128:e240–e327.
12. Sorajja P, Nishimura RA, Gersh BJ, et al. Outcome of mildly symptomatic or asymptomatic obstructive hypertrophic cardiomyopathya long-term follow-up study. J Am Coll Cardiol. 2009; 54:234–241.
13. Cerqueira MD, Weissman NJ, Dilsizian V, et al. Standardized myocardial segmentation and nomenclature for tomographic imaging of the heart a statement for healthcare professionals from the cardiac imaging committee of the council on clinical cardiology of the american heart association. Circulation. 2002; 105:539–542.
14. Schwitter J, Wacker CM, van Rossum AC, et al. MR-IMPACT: comparison of perfusion-cardiac magnetic resonance with single-photon emission computed tomography for the detection of coronary artery disease in a multicentre, multivendor, randomized trial. Eur Heart J. 2008; 29:480–489.
15. Chung SY, Lee KY, Chun EJ, et al. Comparison of stress perfusion mri and spect for detection of myocardial ischemia in patients with angiographically proven three-vessel coronary artery disease. AJR Am J Roentgenol. 2010; 195:356–362.
16. Harrigan CJ, Peters DC, Gibson CM, et al. Hypertrophic cardiomyopathy: quantification of late gadolinium enhancement with contrast-enhanced cardiovascular mr imaging. Radiology. 2011; 258:128–133.
17. Sakamoto T, Amano K, Hada Y, et al. Asymmetric apical hypertrophy: ten years experience. Postgrad Med J. 1986; 62:567–570.
18. Eriksson MJ, Sonnenberg B, Woo A, et al. Long-term outcome in patients with apical hypertrophic cardiomyopathy. J Am Coll Cardiol. 2002; 39:638–645.
19. Matsubara K, Nakamura T, Kuribayashi T, Azuma A, Nakagawa M. Sustained cavity obliteration and apical aneurysm formation in apical hypertrophic cardiomyopathy. J Am Coll Cardiol. 2003; 42:288–295.
20. Rakusan K, Flanagan MF, Geva T, Southern J, Van Praagh R. Morphometry of human coronary capillaries during normal growth and the effect of age in left ventricular pressure-overload hypertrophy. Circulation. 1992; 86:38–46.
21. Krams R, Kofflard M, Duncker D, et al. Decreased coronary flow reserve in hypertrophic cardiomyopathy is related to remodeling of the coronary microcirculation. Circulation. 1998; 97:230–233.
22. von Dohlen TW, Prisant LM, Frank MJ. Significance of positive or negative thallium-201 scintigraphy in hypertrophic cardiomyopathy. Am J Cardiol. 1989; 64:498–503.
23. Cannon RO 3rd, Dilsizian V, O'Gara PT, et al. Myocardial metabolic, hemodynamic, and electrocardiographic significance of reversible thallium-201 abnormalities in hypertrophic cardiomyopathy. Circulation. 1991; 83:1660–1667.
24. Lee KH, Jang HJ, Lee SC, et al. Myocardial thallium defects in apical hypertrophic cardiomyopathy are associated with a benign prognosis. Int J Cardiovasc Imaging. 2003; 19:381–388.
25. Panting JR, Gatehouse PD, Yang G-Z, et al. Abnormal subendocardial perfusion in cardiac syndrome X detected by cardiovascular magnetic resonance imaging. N Engl J Med. 2002; 346:1948–1953.
26. Lanza GA. Cardiac syndrome X: a critical overview and future perspectives. Heart. 2007; 93:159–166.
27. Yamada M, Elliott P, Kaski J, et al. Dipyridamole stress thallium-201 perfusion abnormalities in patients with hypertrophic cardiomyopathy. Relationship to clinical presentation and outcome. Eur Heart J. 1998; 19:500–507.
28. Dilsizian V, Bonow RO, Epstein SE, Fananapazir L. Myocardial ischemia detected by thallium scintigraphy is frequently related to cardiac arrest and syncope in young patients with hypertrophic cardiomyopathy. J Am Coll Cardiol. 1993; 22:796–804.
29. O'Hanlon R, Grasso A, Roughton M, et al. Prognostic significance of myocardial fibrosis in hypertrophic cardiomyopathy. J Am Coll Cardiol. 2010; 56:867–874.
30. Cecchi F, Sgalambro A, Baldi M. Microvascular dysfunction, myocardial ischemia, and progression to heart failure in patients with hypertrophic cardiomyopathy. J Cardiovasc Transl Res. 2009; 2:452–461.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download