Abstract
Among exocrine pancreatic tumors, adenosquamous carcinoma is a rare, aggressive subtype with a poor prognosis and a high potential for metastases compared with its more conventional glandular counterpart, adenocarcinoma of the pancreas. We herein describe the imaging findings of pancreatic adenosquamous cell carcinoma with solitary liver metastasis showing different imaging features and also review the previous literature to recognize characteristic imaging features of pancreatic adenosquamous cell carcinoma.
Pancreatic cancer is one of the most lethal malignancies in the world and is the fourth highest cause of cancer deaths in the United States. According to the 2008 annual report of cancer statistics in Korea, malignant pancreatic cancer has the ninth highest incidence rate with a relative 5-year survival rate of 7.6%. The most common pancreatic histologic subtype is adenocarcinoma, which has a poor survival rate and accounts for 80-85% of pancreatic tumors. In contrast, adenosquamous cell carcinoma is a very rare and more aggressive subtype that accounts for 1-4% of all exocrine malignancies of the pancreas (1).
The prognosis of pancreatic adenosquamous cell carcinoma is worse than that of invasive ductal adenocarcinoma: merely 1.6-9.8 months. Multidisciplinary treatments including aggressive surgery, intraoperative radiation therapy, and locoregional chemotherapy have also been reported to improve the survival of patients to inhibit liver metastasis and local recurrence especially for advanced unresectable or metastatic tumors. Among these treatments, surgical resection offers the only possibility for improved survival in resectable tumors, statistically significantly (2). Nontheless, the overall survival is more dismal than that of invasive adenocarcinoma. Although adenosquamous carcinoma of the pancreas is a rare subtype, it is important to accurately diagnose it based on radiologic findings considering its dismal prognosis and evaluate the operability.
Here, we report the imaging findings related to pancreatic adenosquamous cell carcinoma with solitary liver metastasis in a 56-year-old patient and review the literature.
A 56-year-old man was admitted to our hospital with a complaint of epigastric pain and dyspepsia that had developed 2 weeks previously. A laboratory examination revealed that his amylase and lipase levels were moderately elevated at 132 IU/L (0-100 IU/L) and 64 U/L (13-60 U/L), respectively, while his erythrocyte sedimentation rate and C-reactive protein level were minimally elevated at 66 mm/h (0-9 mm/hr) and 0.340 mg/dL (0.02-0.3 mg/dL), respectively. Additionally, the serum tumor markers carcinoembryonic antigen and carbohydrate antigen 19-9 were moderately elevated to 6.2 ng/mL (0-5 ng/ml) and 78.4 IU/mL (0-37 IU/ml), respectively. However, the patient's alpha-fetoprotein level, complete blood count, and liver function tests were normal.
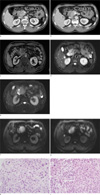
For further evaluation, the patient underwent abdominal computed tomography (CT). In the head and uncinate process of the pancreas, about 4 cm size ill defined heterogenous hypodense mass was observed invading the duodenal wall (Fig. 1a). The main pancreatic duct and distal common bile duct appeared normal. Around the pancreatic mass, several enlarged lymph nodes were observed. Additionally, about 1.4 cm sized, low-attenuation nodule with suspicious perihepatic fibrostreaky density was noted in segment 5 of the liver (Fig. 1b). On the same day, the patient also underwent abdominal magnetic resonance imaging (MRI) with gadolinium ethoxybenzyl diethylenetriamine pentaacetic acid. In the pancreas head and uncinate process, a mass of approximately 4 cm was noted, and it showed a heterogeneous, hyposignal intensity on T1-weighted contrast enhanced MRI(TR2, TE1, GR/FS) (Fig. 1c). An area with high-signal intensity, approximately 1 cm in size, in segment 5 of the liver was noted on a T2-weighted image (TR 800, TE 95, HASTE), which was suggestive of a necrotic nodule (Fig. 1d), whereas the pancreatic tumor itself showed heterogeneous slight high SI on T2 image (Fig. 1e). And on DWI, two masses showed diffusion restrictioned (Fig. 1f, g).
On the following day, positron emission tomography/CT was performed, which showed a conglomerated hypermetabolic mass in the pancreas with a focal hypermetabolic lesion in the adjacent lymph nodes and liver segment 5. Three days after admission, ultrasound-guided biopsy was performed on the hepatic nodule and the pancreatic mass. Histologic evaluation indicated that both the pancreatic mass and the liver nodule were adenosquamous carcinomas, which were likely the result of metastasis from the pancreas. Although we got biopsy specimen, it showed both squamous (Fig. 1h) and adenocarcinoma (Fig. 1i) components.
Although chemotherapy was administered, the primary pancreatic tumor increased in size, and multiple hepatic hypodense metastases were noted on a CT scan performed during a 6-month follow-up.
A few theories have been suggested concerning the formation of adenosquamous carcinoma. The first and most widely accepted theory suggests that preexisting adenocarcinoma undergoes transformation into squamous cell carcinoma (3, 4). Next, the squamous metaplasia theory suggests that squamous metaplasia occurs as a result of ductal inflammation due to chronic pancreatitis or obstruction by an adenomatous tumor resulting in transformation into a malignant adenosquamous pancreatic tumor. Finally, the differentiation theory suggests that primitive cells capable of differentiating into either squamous or glandular cells undergo malignant change.
A pathological diagnosis can be made using percutaneous needle aspiration. Rahemtullah et al. reported that the cytological features derived from a fine needle aspiration biopsy can be used to diagnose pancreatic adenosquamous cell carcinoma (5). In our case, the diagnosis of pancreatic adenosquamous carcinoma with hepatic metastasis was also based on percutaneous needle aspiration biopsy.
No distinctive features of adenosquamous carcinoma that allow it to be distinguished from ductal adenocarcinoma and are observable on imaging studies have been reported until now. However, several articles have reported some common findings that appear to be characteristic features of adenosquamous carcinoma. Nabae et al. concluded that the presence of central necrosis in a large, infiltrative pancreatic tumor is suggestive of pancreatic adenosquamous carcinoma (4). Consistent with this, Zhang and Gao also indicated that features suggestive of adenosquamous carcinoma include large infiltrative lesions and central necrosis visible on CT scans (6). Accordingly, it is thought that squamous cell carcinomas are more likely to be necrotic than adenocarcinomas because angiogenesis cannot catch up with the growth of the tumor (8). Thus, the necrotic portion likely corresponds to the squamous cell carcinoma component. This necrotic, cystic lesion is observable as an area of high-signal intensity on T2-weighted MRI, which is characteristic of adenosquamous cell carcinoma (including squamous cell carcinomas) (8).
The images of adenosquamous carcinoma seem to be similar to those of squamous cell carcinoma with features including hypervascularity and tumor brush on angiography (9), round enhancement of the tumor on contrast CT, and cystic lesions. Nabae et al. also suggested that pancreatic adenosquamous carcinoma should be considered in the differential diagnosis of any hypervascular pancreatic tumor (4). Komatsuda et al. noted the characteristic finding of selective marked portal system invasion without arterial invasion. Thus, peripancreatic vessels should also be carefully observed to uncover characteristic signs of this rare tumor (9).
Overall, a relatively large tumor size, hypervascularity, round enhancement of the tumor on contrast CT, and an internal cystic or necrotic portion could be suggestive of pancreatic adenosquamous cell carcinoma. Until 2011, only 7 cases of adenosquamous carcinoma of the pancreas have been reported in Korea (10). Of these, 6 cases involved metastasis. The sites of metastasis were adjacent organs such as the duodenum, stomach, colon, spleen, or adrenal gland. Rapid locoregional spread with a predominance of neural invasion and distant metastasis is also characteristic of adenosquamous carcinoma (3).
In this case, we identified 2 imaging findings that were different from those of previous reports. First, pancreatic adenosquamous carcinoma consisted of a solid, hypodense, large mass without evidence of necrosis. Second, the metastatic liver nodule was a solitary nodule that had undergone necrotic change, with different imaging features from the primary one. That is the pancreatic mass has more adenocarcinoma component whereas metastatic liver nodule has more squamous component. And also there is no pancreatic ductal dilatation, which is usual finding of pancreatic adenocarcinoma, can be a differential point suggesting pancreatic adenosquamous carcinoma.
In conclusion, we present a case of a 56-year-old male patient with adenosquamous carcinoma of the pancreas. It is a rare case of solid pancreatic tumor with solitary and necrotic hepatic metastasis. This case presented differently than previously reported cases, which encourages us to heighten our awareness of pancreatic adenosquamous carcinoma in order to diagnose it properly.
Figures and Tables
 | Fig. 1
a. In the pancreas head and uncinate process, a relatively ill-defined, low-attenuation mass invading the duodenal wall shows poor enhancement on contrast enhanced image. There is no evidence of tumor necrosis. b. A solitary, hypodense, metastatic nodule in segment 5 of the liver is noted on an enhanced CT scan. c. On a contrast-enhanced T1-weighted image of arterial (20s) phase, mass in the pancreas shows heterogeneous enhancement. d. A solitary hepatic metastatic nodule appears as an area of high-signal intensity on a T2-weighted image, suggesting that the tumor has undergone necrotic change. e. The pancreatic mass itself shows heterogeneous slight high SI on T2-weighted image. f, g. The pancreas and the liver masses shows high SI on high b value image with diffusion restrictioned. h. Hematoxylin and eosin (H & E) staining of a pancreatic adenosquamous carcinoma with a glandular component shows ductal and glandular structures (× 400). i. H & E staining of a pancreatic adenosquamous carcinoma with a squamous component shows an intercellular bridge (× 400). |
References
1. Lampropoulos P, Filippous G, Skafida E, Vasilakaki T, Paschalidis N, Rizos S. Adenosquamous carcinoma of the pancreas, a rare tumor entity: a case report. Cases J. 2009; 2:9129.
2. Trikudanathan G, Dasanu CA. Adenosquamous carcinoma of the pancreas: a distinct clinicopathologic entity. South Med J. 2010; 103:903–910.
3. Ito K, Fujita N, Noda Y, et al. Pancreatic adenosquamous carcinoma, 9mm in size, diagnosed preoperatively by transpapillary biopsy: report of a case. Dig Endosc. 2003; 15:235–239.
4. Nabae T, Yamaguchi K, Takahata S, et al. Adenosquamous carcinoma of the pancreas: report of two cases. Am J Gastroenterol. 1998; 93:1167–1170.
5. Rahemtullah A, Misdraji J, Pitman MB. Adenosquamous carcinoma of the pancreas: cytologic features in 14 cases. Cancer. 2003; 99:372–378.
6. Zhang B, Gao SL. Education of imaging. Hepatobiliary and pancreatic: adenosquamous carinoma of the pancreas. J Gastroenterol Hepatol. 2008; 23:820.
7. Charbit A, Malaise EP, Tubiana M. Relation between the pathological nature and the growth rate of human tumors. Eur J Cancer. 1971; 7:307–315.
8. Inoue T, Nagao S, Tajima H, et al. Adenosquamous pancreatic cancer producing parathyroid hormone-related protein. J Gastroenterol. 2004; 39:176–180.
9. Komatsuda T, Ishida H, Konno K, et al. Adenosquamous carcinoma of the pancreas: report of two cases. Abdom Imaging. 2000; 25:420–423.
10. Na YJ, Shim KN, Cho MS, et al. Primary adenosquamous cell carinoma of the pancreas: a case report with a review of the Korean literature. Korean J Intern Med. 2011; 26:348–351.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download