Abstract
A 66-year-old woman was transferred to our hospital due to her right breast cancer. Preoperative breast MRI shows 1.9 cm malignancy on her right breast (cT1N0M0) and incidentally found osteosclerotic change of left coststernoclavicular region. Bone scintigraphy showed hot uptake and the possibility of bone metastasis was not excluded. However, because the bone metastasis is not common in early stage cancer and the costosternoclavicular region is not common site, other possibility should be considered. SAPHO syndrome can be diagnosed even in the absence of dermatosis when there is an axial or appendicular osteitis and hyperostosis, especially in costosternoclavicular region. Though breast imaging specialists are not accustomed to this disease entity, awareness and diagnosis of the SAPHO syndrome can help differentiate bone metastasis.
Bone is the most common site to which breast cancer metastasizes (1). Bone represents the first site of metastasis for 26~50% of patients with metastatic breast cancer (2, 3). If hot uptake in a patient with breast cancer is noted on the bone scintigraphy, the possibility of bone metastasis should be considered. However, because metastatic arthritis rarely occurs and the costosternoclavicular region is not common site for metastasis, other possibility should be considered such as SAPHO syndrome; it is an acronym of syndrome of synovitis, acne, pustulosis, hyperostosis, and osteitis (4) and its diagnosis can be made on the base of the solely musculoskeletal system without even skin lesions. And the costosternoclavicular region is known to be typical site of it (4). So, if the sclerotic and hyperostotic bone change of costosternoclavicular region was incidentally found in early breast cancer patient, try to include the SAPHO syndrome as a possible differential diagnosis. Here, we present a patient with right breast cancer and incidentally found sclerotic and hyperostotic bony lesion of her left costosternoclavicular region, which was confirmed as chronic active inflammation without causative organism.
A 66-year-old woman was referred to our hospital due to breast abnormality detected on screening examination. Mammography shows heterogeneously dense parenchyma without any mass or microcalcifications. Breast ultrasound was done to find about 1.3 cm sized irregular mass (Fig. 1a) at her right breast which was proven as an invasive ductal carcinoma via gun biopsy. For preoperative evaluation, dynamic contrast enhanced breast MRI was done and about 1.9 cm enhancing round mass was seen on MR images. Simultaneously, osteosclerotic change and mild marrow signal change of her left costosternoclavicular region were encountered on T1- and T2-weighted images (Fig. 1b). Moreover, bone scintigraphy showed hot uptake and the possibility of bone metastasis was suggested (Fig. 1c). However, primary breast malignancy was in its early stage (less than 2 cm on the MRI which was T1 stage and without evidence of ipsilateral axillary lymph node metastasis, cT1N0M0), and the costosternoclavicular region was not a common site of metastasis. Her serum alkaline phosphatase was 103 IU/L which was within normal range (35~104 IU/L) and serum calcium and phosphorus were within normal range, too. So, another possible diagnosis should be sought such as SAPHO syndrome and the possibility of bone metastasis was thought to be low. To obtain the pathology, computed tomography (CT) guided bone biopsy was done using 14 gauge bone biopsy needle (Ostycut®, Bard Biopsy Systems, Tempe, AZ, USA) (Fig. 1d) and chronic active inflammation without causative organism was confirmed (Fig. 1e). After this procedure, she underwent right lumpectomy for invasive ductal carcinoma. She underwent follow up bone scintigraphy after 1 year and positron emission tomography-CT (PET-CT) after 2 years and uptake of the left costosternoclavicular region has no change at all. Follow up serology for alkaline phosphatase, serum calcium and phosphorus after 2 years later were within normal range, too.
Bone is known to be the most common site to which breast cancer metastasizes (1). Between 30~85% of patients with metastatic breast cancer will develop bone metastases during the course of the disease. Bone also represents the first site of metastasis for 26~50% of patients with metastatic breast cancer (2, 3). To find out bone metastasis is important because therapeutic plans can be changed and complications of bone metastasis such as bone pain, pathologic fracture, hypercalcemia, and spinal cord compression can profoundly impair the quality of life (5). So, to discern between bone metastasis and others is also important to avoid unnecessary treatment and to do proper treatment as soon as possible. Breast cancer preferentially metastasizes to vertebrae and the pelvis, followed by ribs, skull, and femur, probably because the vertebrae are highly vascularized and contain 75% of the body's red marrow (1). On MRI, normal bone marrow shows a high signal intensity on T1-weighted image, whereas metastases appear as areas of reduced signal, reflecting the replacement of fat in the marrow by the tumor (1). In our case, the marrow signal change on T1-weighted image is minimal and sclerotic and hyperostotic change is a main finding.
Bone scintigraphy is known to be the most commonly used tool to detect bone metastasis (3). It visualizes increases in osteoblastic activity and skeletal vascularity. The sensitivity and specificity of bone scintigraphy are variable, ranging from 62 to 100% in sensitivity and 78 to 100% in specificity. And it has high false positive rate than X-ray, hot uptakes can occur in several disease processes such as neoplasia, trauma and inflammation.
Meanwhile screening for bone metastasis is generally thought to be unnecessary if the primary breast malignancy is at an early stage. Once a clinical justification (pain or biomarker elevation) is made for screening, bone scintigraphy is advisable for the first-line imaging modality because of its high sensitivity. If bone scintigraphy findings are abnormal, X-ray can be added to assess the degree of bone loss and risk of pathologic fracture and to establish a baseline for comparison with future treatment assessments. Due to variable specificity of bone scintigraphy, findings of solitary lesion, diffuse involvement, and photon-deficient lesions should be examined further by X-ray and especially in solitary lesion, biopsy is recommended. In our case, bone scintigraphy shows solitary uptake in her left costosternoclavicular region even without biomarker elevation which was one of indications of biopsy (1). CT guided bone biopsy was done to confirm chronic active inflammation without any causative organism. Although SAPHO syndrome is known to be a constellation of complex clinical and imaging manifestations, the histologic features of osseous manifestation were reported in nine cases (6). The histologic features varied but seemed related to the duration of the patients' musculoskeletal symptoms. Early lesions contained acute inflammation, edema, and prominent periosteal bone formation, histologically indistinguishable from ordinary bacterial osteomyelitis, whereas late lesions demonstrated markedly sclerotic bone trabeculae with prominent marrow fibrosis and only mild chronic inflammation. The histologic findings in SAPHO syndrome are variable and nonspecific and may depend on the duration of disease, but it is important to recognize the spectrum of histologic changes possible in the syndrome and to realize that clinicopathologic correlation is necessary to avoid misdiagnosis and unnecessary long-term antibiotic therapy. In our case, the pathology seems to be in its chronic stage.
SAPHO syndrome is an acronym for an increasingly recognized syndrome of synovitis, acne, pustulosis, hyperostosis and osteitis (1). With the increase in reports dealing with growing numbers of patients with SAPHO syndrome, this disease entity should be suspected in patients who fulfill one of the following diagnostic criteria (7): ① Osteoarticular manifestations with dermatosis like acne conglobate, acne fulminans, or palmoplantar pustulosis; ② axial or appendicular osteitis and hyperostosis with or without dermatosis; ③ chronic recurrent multiple osteomyelitis involving the axial or appendicular skeleton with or without dermatosis.
Though the definitions of osteoarticular and dermatologic manifestations are fundamental to diagnose the SAPHO syndrome, the musculoskeletal manifestation is essential. The skin and osteoarticular manifestations of the SAPHO syndrome do not necessarily parallel one another or even in the absence of any documented skin lesions, if the characteristic bone lesions are present, the diagnosis of SAPHO syndrome is possible (8). Skin eruption can begin within 2 years before or after the osteoarthritis in 70% of patients, but latency periods of more than a decade have been reported (9).
The dermatologic conditions associated with the SAPHO syndrome generally fall into two categories, either acne or pustulosis. Of them, the palmoplantar pustulosis is known to be the prototypical skin lesion associated with SAPHO syndrome. In general population, the prevalence of palmoplantar pustulosis is estimated to be about 0.05%. However, of those patients who have palmoplantar pustulosis, the prevalence of osteoarthritis is variable, ranging from 10 to 30%. In the subgroup of palmoplantar pustulosis patients with osteoarthritis, the favored target area is known to be the upper anterior chest wall, ranging from 60 to 95% of the patients. In our case, the patient did not suffer any dermatologic symptoms such as acne or palmoplantar pustolosis even after 2 year later. However, the cause and pathophysiology of the SAPHO syndrome are poorly understood, putative hypotheses have been proposed related to an autoimmune response that could be triggered by a bacterial or viral pathogen (10). Costosternoclavicular hyperostosis is characterized by bone hypertrophy and ligamentous ossification involving the anterior chest wall (the sternum, upper anterior ribs, and clavicle). The typical patient is in the 4th to 6th decades of life and experiences pain, tenderness, and swelling in the upper portion of the anterior chest wall bilaterally. About two thirds of patients with costosternoclavicular hyperostosis will experience characteristic skin condition such as palmoplantar pustulosis (50%), psoriasis vulgaris (10%) and severe acne (10%). Radiographically, the dominant abnormality is hyperostosis in the region of the sternum, upper ribs, and clavicles. CT can be helpful when findings include an isolated periosteal reaction involving the clavicles, isolated arthritis of the sternoclavicular joint, and isolated erosion and sclerotic processes of the manubriosternal joint. Isolated erosion and sclerotic processes of costosternoclavicular region are identical to ours.
Awareness and diagnosis of the SAPHO syndrome are important because such awareness facilitates differentiation from other entities that can produce similar imaging findings but have very different treatments and prognoses, such as metastasis. Although bone is the most common site to which breast cancer metastasizes, by considering the SAPHO syndrome in the differential diagnosis of sclerotic and hyperostotic bones, especially in costosternoclavicular region, radiologist may avoid unnecessary diagnostic and therapeutic delay or error. And the patient can be comforted in knowing that their affliction generally has a benign and self-limited course.
Figures and Tables
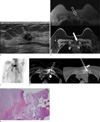
Fig. 1
Imaging findings in a 66-year-old woman with her right breast cancer.
a. On breast ultrasound, about 1.3 cm-sized irregular mass is seen (hollow arrow) at her right breast which was confirmed as an invasive ductal carcinoma via gun biopsy. b. Breast MRI shows sclerotic bone changes of her left sternoclavicular joint (hollow arrows). On T2 weighted image, mild high signal intensities on the marrows of her left clavicle and manubrium (hollow arrow) are accompanied (top image) and on T1 weighted image, sclerotic cortical change is seen (white arrow, bottom image). c. Bone scintigraphy shows hot uptake at left costosternoclavicular region (hollow arrow). d. The osteosclerotic changes of left sternoclavicular region are seen on CT (right side, hollow arrow). To obtain the pathology, a bone biopsy needle (left side, white arrow) was advanced under CT guidance and chronic active inflammation without causative organism was confirmed. e. The chondro-osseous tissue is infiltrated by many inflammatory cells mainly composed of neutrophils and lymphocytes (× 100, H & E stain).
References
1. Hamaoka T, Madewell JE, Podoloff DA, Hortobagyi GN, Ueno NT. Bone imaging in metastatic breast cancer. J Clin Oncol. 2004; 22:2942–2953.
2. Coleman RE, Rubens RD. The clinical course of bone metastases from breast cancer. Br J Cancer. 1987; 55:61–66.
3. Hortobagyi GN. Bone metastases in breast cancer patients. Semin Oncol. 1991; 18:11–15.
4. Boutin RD, Resnick D. The sapho syndrome: an evolving concept for unifying several idiopathic disorders of bone and skin. AJR Am J Roentgenol. 1998; 170:585–591.
5. Rubens RD. Bone metastases--the clinical problem. Eur J Cancer. 1998; 34:210–213.
6. Reith JD, Bauer TW, Schils JP. Osseous manifestations of sapho (synovitis, acne, pustulosis, hyperostosis, osteitis) syndrome. Am J Surg Pathol. 1996; 20:1368–1377.
7. Assmann G, Simon P. The sapho syndrome--are microbes involved? Best Pract Res Clin Rheumatol. 2011; 25:423–434.
8. Chamot AM, Vion B, Gerster JC. Acute pseudoseptic arthritis and palmoplantar pustulosis. Clin Rheumatol. 1986; 5:118–123.
9. Sonozaki H, Mitsui H, Miyanaga Y, et al. Clinical features of 53 cases with pustulotic arthro-osteitis. Ann Rheum Dis. 1981; 40:547–553.
10. Hellmann DB. Spondyloarthropathy with hidradenitis suppurativa. JAMA. 1992; 267:2363–2365.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download