Abstract
Purpose
To evaluate the correlation of lesion-to-normal ratio (LNR) of signal intensity from double inversion recovery MR imaging and total choline-containing compound (tCho) resonance from single voxel MR spectroscopy in breast cancers.
Materials and Methods
Between August 2008 and December 2009, 28 patients who were diagnosed as breast cancer and had undergone both double inversion recovery (DIR) MR imaging and MR spectroscopy (MRS) were included in this study. The signal intensities of the lesion (L) and ipsilateral normal breast tissue (N) were measured in region of interest of each breast cancer in DIR and contrast enhance MR image (CE-T1WI) to calculate the LNR value for each technique. MRS was performed using single-voxel MR spectroscopy. The height, width and area of tCho resonance were compared with each LNR of DIR and CE-T1WI. We used Pearson's correlation coefficient (r) for correlation analysis and the significance level was p=0.05.
Results
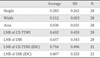
There was no statistically significant correlation between LNR of CE-T1WI and height (r=-0.322, p=0.094), width (r=-0.233, p=0.232) and area (r=-0.309, p=0.109) of MRS tCho. There was no statistically significant correlation between LNR of DIR and height (r=0.067, p=0.735), width (r=-0.287, p=0.139) and area (r=0.012, p=0.953) of MRS tCho, either. The Pearson's correlation coefficient was 0.186 between LNRs of CE-T1WI and DIR (p=0.344).
Figures and Tables
Fig. 1
Representative double inversion recovery (DIR) image and MRS spectrum on a patient with invasive ductal carcinoma in 47-year-old woman.
a. About 3.0 cm sized rim enhancing oval mass was noted at upper central portion of the right breast in sagittal scan of contrast-enhanced T1-weighted imaging (CE-T1WI). b. In sagittal DIR image, the mass shows the bright high signal intensity compared with that of normal parenchyma which results the lesion more conspicuous. c. Single-voxel MR spectroscopy reveals prominent tCho resonance peak at 3.2ppm. The height, width and area of the tCho were 1.068, 0.1 and 0.120, respectively.
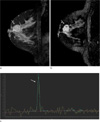
Fig. 2
Representative double inversion recovery (DIR) image and MRS spectrum on a patient with mucinous carcinoma in 66-year-old woman.
a. About 2.5 cm sized heterogenous enhancing mass was shown at the left central breast in sagittal contrast-enhanced T1-weighted imaging (CE-T1WI). b. Axial scan of DIR image represents rather iso- to low signal intensity mass at the left central breast. c. At 3.2ppm resonance, a striking tCho peak was visualized with the height, width and area as 0.526, 0.11 and c 0.065, respectively.
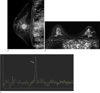
Table 2
Result of Height, Width, Area of the Total Choline-containing Compound (tCho) Resonance Peak and the Lesion-to-normal Ratio (LNR) of Contrast-enhanced T1-weighted Imaging (CE-T1WI) and Double Inversion Recovery (DIR)
References
1. Huang W, Fisher PR, Dulaimy K, Tudorica LA, O'Hea B, Button TM. Detection of breast malignancy: diagnostic MR protocol for improved specificity. Radiology. 2004; 232:585–591.
2. Saslow D, Boetes C, Burke W, et al. American Cancer Society guidelines for breast screening with MRI as an adjunct to mammography. CA Cancer J Clin. 2007; 57:75–89.
3. Yen YF, Han KF, Daniel BL, et al. Dynamic breast MRI with spiral trajectories: 3D versus 2D. J Magn Reson Imaging. 2000; 11:351–359.
4. Sinha S, Sinha U. Functional magnetic resonance of human breast tumors. Ann N Y Acad Sci. 2002; 980:95–115.
5. Liberman L, Morris EA, Lee MJY, et al. Breast lesions detected on MR imaging: features and positive predictive value. AJR Am J Roentgenol. 2002; 179:171–178.
6. Orel SG, Schnall MD. MR imaging of the breast for the detection, diagnosis, and staging of breast cancer. Radiology. 2001; 220:13–30.
7. Gruber S, Debski BK, Pinker K, et al. Three-dimensional proton MR spectroscopic imaging at 3 T for the differentiation of benign and malignant breast lesions. Radiology. 2011; 261:752–761.
8. Yabuuchi H, Matsuo Y, Okafuji T, et al. Enhanced mass on contrast-enhanced breast MR imaging: lesion characterization using combination of dynamic contrast-enhanced and diffusion-weighted MR images. J Magn Reson Imaging. 2008; 28:1157–1165.
9. Kim JH, Ryu JK, Jahng GH, Song JY. Double inversion recovery MR imaging of the breast: efficacy in detection of breast cancer. J Magn Reson Imaging. 2014; 39:51–58.
10. Bartella L, Morris EA, Dershaw DD, et al. Proton MR spectroscopy with choline peak as malignancy marker improves positive predictive value for breast cancer diagnosis: preliminary study. Radiology. 2006; 239:686–692.
11. Meisamy S, Bolan PJ, Baker EH, et al. Adding in vivo quantitative 1H MR spectroscopy to improve diagnostic accuracy of breast MR imaging: preliminary results of observer performance study at 4.0 T. Radiology. 2005; 236:465–447.
12. Jacobs MA, Barker PB, Bottomley PA, Bhujwalla Z, Bluemke DA. Proton magnetic resonance spectroscopic imaging of human breast cancer: a preliminary study. J Magn Reson Imaging. 2004; 19:68–75.
13. Sardanelli F, Fausto A, Di Leo G, de Nijs R, Vorbuchner M, Podo F. In vivo proton MR spectroscopy of the breast using the total choline peak integral as a marker of malignancy. AJR Am J Roentgenol. 2009; 192:1608–1617.
14. Bartella L, Huang W. Proton (1H) MR Spectroscopy of the Breast. Radiographics. 2007; 27:S241–S252.
15. Shin HJ, Baek HM, Ahn JH, et al. Prediction of pathologic response to neoadjuvant chemotherapy in patients with breast cancer using diffusion-weighted imaging and MRS. NMR Biomed. 2012; 25:1349–1359.
16. Turetschek K, Wunderbaldinger P, Bankier AA, et al. Double inversion recovery imaging of the brain: initial experience and comparison with fluid attenuated inversion recovery imaging. Magn Reson Imaging. 1998; 16:127–135.
17. Redpath T, Smith F. Technical note: use of a double inversion recovery pulse sequence to image selectively grey or white brain matter. Br J Radiol. 1994; 67:1258.
18. Meara SJP, Barker GJ. Evolution of the longitudinal magnetization for pulse sequences using fast spin echo readout: application to fluid attenuated inversion recovery and double inversion recovery sequences. Magn Reson Med. 2005; 54:241–245.
19. Rakow-Penner R, Daniel B, Yu H, Sawyer GA, Glover GH. Relaxation times of breast tissue at 1.5 T and 3T measured using IDEAL. J Magn Reson Imaging. 2006; 23:87–91.
20. Males RG, Vigneron DB, Star-Lack J, et al. Clinical application of BASING and spectral/spatial water and lipid suppression pulses for prostate cancer staging and localization by in vivo 3D 1H magnetic resonance spectroscopic imaging. Magn Reson Med. 2000; 43:17–22.
21. Lauenstein TC, Sharma P, Hughes T, Heberlein K, Tudorascu D, Martin DR. Evaluation of optimized inversion-recovery fat suppression techniques for T2-weighted abdominal MR imaging. J Magn Reson Imaging. 2008; 27:1448–1454.
22. Begley JK, Redpath TW, Bolan PJ, Gilbert FJ. In vivo proton magnetic resonance spectroscopy of breast cancer: a review of the literature. Breast Cancer Res. 2012; 14:207–216.
23. Podo F. Tumour phospholipid metabolism. NMR Biomed. 1999; 12:413–443.
24. Katz-Brull R, Seger D, Rivenson-Segal D, Rushkin E, Degani H. Metabolic markers of breast cancer: enhanced choline metabolism and reduced choline-ether phospholipid synthesis. Cancer Res. 2002; 62:1966–1970.
25. Su MY, Baik HM, Yu HJ, Chen JH, Mehta RS, Nalcioglu O. Comparison of choline and pharmacokinetic parameters in breast cancer measured by MR spectroscopic imaging and dynamic contrast enhanced MRI. Technol Cancer Res Treat. 2006; 5:401–410.
26. Baek HM, Yu HJ, Chen JH, Nalcioglu O, Su MY. Quantitative correlation between (1)H MRS and dynamic contrast-enhanced MRI of human breast cancer. Magn Reson Imaging. 2008; 26:523–531.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download