Most soft tissue tumors show high signal on T2-weighted images (T2WIs). Some tumors with the same histologic diagnoses showed different MR appearances in that some had a long T2 while others had a short T2 (1). Abundant collagen and marked hypocellularity or acellularity in a soft-tissue tumor or hemosiderin-laden masses result in a decreased signal on T2WI (1, 2). Lesions with decreased signal intensity (SI) on both T1- and T2WIs might represent densely mineralized lesions. And also thrombus, phlebolith, hemorrhage and fast blood flow can show low signal intensity on T2WI. We defined low SI on T2WI as signal intensity lower than that of fat or fluid and signal intensity equal, slightly higher or lower to that of skeletal muscle. The aims of this article are to differentiate soft tissue masses showing low SI according to the histopathologic findings, knowing components contributing to low signal intensity on T2WIs.
Fibroma of the tendon sheath is a benign fibroblastic proliferation that occurs in the distal extremities often attached to tendon or tendon sheath (3). The MR imaging findings vary when areas with increased cellularity or myxoid change occur within the lesion (4). Low SI on T2WI can be attributed to the overall hypocelluarity and highly collagenous stroma found in this region (1, 3) (Fig. 1).
Nodular fasciitis is a benign soft tissue lesion characterized by proliferation of fibroblasts (5). Lesions having a myxoid matrix and hypercellularity in the early stage demonstrate higher SI than that of muscle on T1WIs and than that of fat on T2WIs. Mature lesions are often characterized by increased fibrosis, and the fibrotic areas present as markedly hypointense to the surrounding muscles on all pulse sequences (5, 6) (Fig. 2).
Desmoplastic fibroblastoma is a fibrous soft tissue tumor. The low SI on T2WI is attributed to the low cellularity of the mass in a background of abundant collagen (7).
The fibromatoses are a diverse group of a soft tissue lesions that occur at different ages and anatomic locations and that have common histopathologic features (8). Fibromatosis in the proliferative phase demonstrates a high degree of cellularity and have a predominantly hyperintense signal on T2WIs. These lesions typically mature over time with increased collagen content and reduced cellularity, with a resultant decrease in SI on T2WIs (9) (Fig. 3).
MFH is a pleomorphic sarcoma, although MFH now appears more related to the fibroblasts, myofibroblasts, or undifferentiated mesenchymal cells. Generally MFH demonstrates an intermediate SI on T1WIs and heterogeneous high SI on T2WIs reflecting the variable pattern seen histologically (10). These variations include regions with prominent fibrous tissue (high collagen content; low SI); calcification (low signal foci); hemorrhage (high signal on all pulse sequences; fluid levels), necrosis (low signal on T1WI and high signal on T2WIs); and areas of tumor with lower collagen content (intermediate on T1WI; high signal on T2WI) (11) (Fig. 4).
Elastofibroma is a degenerative or reactive fibrous pseudotumor that arises most commonly between the inferior margin of the scapula and posterior chest wall in elderly individuals (9). On both T1- and T2WIs, the lesion is well defined and has intermediate signal intensity similar to that of skeletal muscle, corresponding to areas of dense fibrous connective tissue with interlaced areas of SI similar to that of fat (12) (Fig. 5).
Lipomas occasionally contain other mesenchymal elements. The most common of these is fibrous connective tissue, which may demonstrate a septal configuration, appearing as linear areas of decreased signal on MRI, regardless of pulse sequence (Fig. 6). When significant fibrous tissue is present, these lesions may be termed fibrolipoma.
Traumatic neuromas develop from a nonneoplastic proliferation of the proximal end of a severed, partially transected, or injured nerve as a result of trauma or surgery. They typically have intermediate to high SI on T2WI. Their SI is often heterogeneous, with a ringlike pattern ("fascicular sign"), which correlates with the histologic morphology of nerve fascicles (13) (Fig. 7).
Schwannomas are benign slow-growing encapsulated tumors of nerve sheath origin (13). Histologically they are composed of two cell types: Antoni A and Antoni B. The Antoni A cells are densely packed and arranged in fascicles, Antoni B cells are less compact and are prone to cystic degeneration. T2WIs corresponds histologically to peripheral myxomatous tissue and central fibrocollagenous tissue. Another intrinsic MR imaging characteristic, fascicular sign manifests as multiple small ringlike structures (with peripheral higher signal intensity) on either T2- or proton density-weighted MR images. This sign corresponds to the fascicular bundles seen pathologically in neurogenic neoplasms (13).
Neurofibromas are nonencapsulated, often infiltrative lesions (14). Diffuse type is a poorly defined lesion that spreads along connective tissue septa and surrounds rather than destroys adjacent normal structures. Skin and subcutaneous tissue involvement was most typical (15). They often show predominant low SI on T2WIs, may be related to the high collagen content of these lesions (13). When the central area of the tumor is composed of dense collagenous tissue, it has decreased SI on T2WIs, resulting in a characteristic "target" appearance (14) (Fig. 8).
GCTTS is a benign proliferative lesion of synovial origin arising from the tendon sheath, joint capsule, bursa or ligaments (14). The MR imaging features are variably heterogeneous and depend on the relative proportions of fat, fibrous tissue, and hemosiderin, although the SI of lesions is predominantly low (14, 16). Typical lesions have areas of low SI on both T1- and T2WIs due to the paramagnetic effect of hemosiderin (14, 16).
Synovial osteochondromatosis is a benign monarticular disorder of uncertain cause characterized by proliferation and metaplastic transformation of the synovium with formation of multiple cartilaginous nodules (17). MR imaging is characterized as lobulated, homogeneous, intermediate, intraarticular SI similar to that of muscle on T1WIs, with high signal intensity on T2WIs and focal areas of low SI with all pulse sequences. The areas of signal void corresponded to regions of calcification (18) (Fig. 10).
Myositis ossificans (MO) is a benign, solitary, self-limiting, ossifying soft-tissue mass typically occurring within skeletal muscle. The MR imaging appearance changes with the age of the lesion, reflecting the evolving histologic characteristics. In the intermediate or older lesions, MR images showed curvilinear and irregular regions of decreased SI peripherally as well as within lesions, corresponding to mineralization seen on CT scans and radiographs. Areas of hemosiderin deposition from previous hemorrhage and fibrosis also may contribute to areas of decreased SI on both pulse sequences (19, 20) (Fig. 11).
Intravascular papillary endothelial hyperplasia (IVPEH) is a reactive proliferative lesion of endothelial cells within the vessels and is usually associated with thrombi. Hyperintense region on T2WI corresponded to the ectatic blood-pool space in the hemangioma as well as to isointense central regions composed of thrombosis and endothelial papillary proliferative tissue (22). T2 sequences may show low SI areas reflecting thrombotic or hemorrhagic material and low signal internal septae have been described (23) (Fig. 13).
Hemangioma is one of the most common soft tissue tumors and usually located superficially but may involve deep structures such as skeletal muscle. On T2WI, it shows areas that are very high SI due to vascular tissue and other regions that are intermediate in SI due to fat (24). Punctate or reticular low SI areas may be present, representing fibrous tissue, fast flow within vessels, or foci of calcification. Areas of thrombosis appear as circular low SI areas at MR imaging, similar to phleboliths (25).
Synovial sarcoma is a mesenchymal neoplasm of uncertain pathogenesis. MR images may demonstrate complex signal characteristics, with fluid levels, hemorrhage, and septation in the lesions. Pathologically triple signal appearance on T2WIs reflects the mixture of solid, cystic, fibrous, and hemorrhagic elements present in many synovial sarcomas (26).
Alveolar soft part sarcoma is a highly vascular malignant tumor which occurs most often in the soft tissues of the pelvis and lower limbs. On dynamic MRI, the margin of the tumor enhanced strongly in the early phase and washout occurred in the late phase. The signal void representing fast flow was shown at the margin of the tumor (27) (Fig. 14).
Lymphomas are characterized pathologically by the proliferation of cells native to the lymphoid tissue-lymphocytes, histiocytes, and their precursors (29). At MRI extranodal soft-tissue lymphomas are homogeneously isointense or slightly hypointense relative to normal muscle on T1WIs and hyperintense to muscle on T2WIs (30). Lymphomas usually have homogeneously low SI on T2WIs because of their dense cellularity (31).
In conclusions, We described the possible disease entities that can show low signal intensity on T2-weighted image according to the composition of the soft tissue tumors. In addition to this, characteristic locations, morphology and signal intensities on other sequences may be helpful for the differential diagnosis of mostly nonspecific soft tissue masses on MRI.
Figures and Tables
Fig. 1
61-year-old male patient with a fibroma of the tendon sheath. (a) On a coronal fat suppressed T2-weighted image, the mass located at the lateral aspect of the vastus lateralis muscle shows signal intensity equal to that of skeletal muscle. This finding is attributable to the high quantity of collagen in many of these tumors. (b) A photomicrograph (H & E staining, ×200) shows hypocellularity and the mass contains a dense collagenous matrix and scattered spindle-shaped fibroblasts.
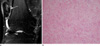
Fig. 2
19-year-old male patient with nodular fasciitis. (a) On a sagittal fat-suppressed T2WI of the forearm, a well-defined soft tissue mass shows signal intensity higher than that of the subcutaneous fat and muscle. (b) A photomicrograph (H & E staining, ×400) shows increased cellularity. There are few foci of myxoid degeneration.
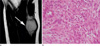
Fig. 3
40-year-old male patient with fibromatosis. (a) A mass located at the anterior chest wall shows signal intensity slightly higher than that of skeletal muscle on a T2WI. The mass contains a region with lower signal intensity. (b) A photomicrograph (H & E staining, ×100) shows a lesion that is predominantly fibrotic with low cellularity and with heavy collagen deposition.

Fig. 4
80-year-old male patient with a malignant fibrous histiocytoma. (a) On an axial T2WI of the right thigh, the mass located at the superficial aspect of the fascia shows intermediate signal intensity higher than that of skeletal muscle. (b) A photomicrograph (H & E staining, ×40) shows hypercellularity with a relatively low collagen content.

Fig. 5
64-year-old male patient with an elastofibroma. (a) An axial T2WI shows soft tissue with signal intensity similar to that of the adjacent skeletal muscle, interlaced with streaks of tissue with signal intensity of fat between the inferior margin of the scapula and posterior chest wall. This appearance corresponds to areas of dense fibrous connective tissue interlaced with areas of fat. (b) A photomicrograph (H & E staining, ×400) demonstrates scattered elastic fibers in a background of collagenous tissue. Relative hypocellularity is seen.
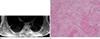
Fig. 6
45-year-old male patient with a fibrolipoma. (a) An axial T2WI shows the nonadipose component to have decreased signal intensity similar to that of muscle. Note the presence of adipose tissue in the interstices of the mass. (b) A photomicrograph (H & E staining, ×100) shows fibrolipomatous proliferation around the nerves (arrowheads). The presence of fibrous septae (arrow) is noted.
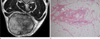
Fig. 7
31-year-old male patient with an amputation neuroma. (a) The mass (arrows) located at the ventral aspect of the distal portion of the amputated forearm shows a fascicular sign on an axial T2WI of the forearm. (b) A photomicrograph (H & E staining, ×100) shows numerous nerve bundles and the surrounding fibroblasts with a collagenous matrix.
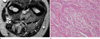
Fig. 8
34-year-old female patient with two neurofibromas. (a) An axial T2WI shows a well-defined mass with a center of low signal intensity and high signal intensity peripheral rim (target sign) through two muscular compartments. (b) A photomicrograph (H & E staining, ×200) shows scattered neurofibroma cells and a background of loosely packed collagen fibers and myxoid stroma.
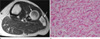
Fig. 9
33-year-old female patient with diffuse-type giant cell tumor(pigmented villonodular synovitis). (a) A fat-suppressed axial T2WI (a) shows a lobulated mass with low signal intensity in the retrocalcaneal bursa. (b) Photomicrographs (H & E staining, ×100) demonstrate the presence of multiple foci of hemosiderin deposits.
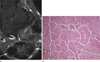
Fig. 10
60-year-old female patient with synovial osteochondromatosis. (a) On a sagittal T2WI of the ankle, multiple areas of signal void are noted within the effusion of the posterior ankle joint. (b) A corresponding plain radiograph shows the presence of multiple calcified nodules.
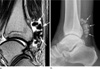
Fig. 11
40-year-old female patient with myositis ossificans. (a) A sagittal T2WI shows an inhomogeneous, well-defined mass surrounded by diffuse edema in the anteromedial aspect of the upper arm. The mass is surrounded by a thin hypointense rim, representing peripheral ossification. (b) As seen on a plain radiograph, a faintly mineralized mass is present.
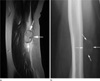
Fig. 12
11-year-old male patient with a pilomatricoma. (a) On a coronal T2WI, a heterogeneous low signal intensity mass is seen in the subcutaneous fat layer of the cheek. (b) A photomicrograph (H & E staining, ×40) shows calcifications and ossification.
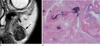
Fig. 13
25-year-old male patient with intravascular papillary endothelial hyperplasia. (a) A coronal T2WI of the thigh shows a well-defined mass with a hyperintense peripheral region and multiple hypointense or isointense central regions. (b) A photomicrograph (H & E staining, ×100) shows hemorrhage and a thrombus (arrows).
Fig. 14
26-year-old male patient with an alveolar soft part sarcoma. A coronal T2WI of the thigh shows a lobulated margined mass with higher signal intensity than that of the skeletal muscle and lower signal intensity than that of fat. Note the serpentine flow voids, representing enlarged blood vessels. Signal voids (arrows) are seen at the margin of the tumor.
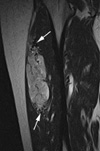
Fig. 15
15-year-old male patient with an epidermoid cyst. (a) A coronal T2WI obtained through the abdominal wall shows a well-defined mass of intermediate to high signal intensity with dot-like low signal components in the subcutaneous fat layer. (b) A photomicrograph (H & E staining, ×100) shows keratin debris within the epithelial lining.

References
1. Sundaram M, McGuire MH, Schajowicz F. Soft-tissue masses: histologic basis for decreased signal (short T2) on T2-weighted MR images. AJR Am J Roentgenol. 1987; 148:1247–1250.
2. Sundaram M, McLeod RA. MR imaging of tumor and tumorlike lesions of bone and soft tissue. AJR Am J Roentgenol. 1990; 155:817–824.
3. Hur J, Damron TA, Vermont AI, Mathur SC. Fibroma of tendon sheath of the infrapatellar fat pad. Skeletal Radiol. 1999; 28:407–410.
4. Fox MG, Kransdorf MJ, Bancroft LW, Peterson JJ, Flemming DJ. MR imaging of fibroma of the tendon sheath. AJR Am J Roentgenol. 2003; 180:1449–1453.
5. Wang XL, De Schepper AM, Vanhoenacker F, et al. Nodular fasciitis: correlation of MRI findings and histopathology. Skeletal Radiol. 2002; 31:155–161.
6. Blacksin MF, Ha DH, Hameed M, Aisner S. Superficial softtissue masses of the extremities. Radiographics. 2006; 26:1289–1304.
7. Miettinen M, Fetsch JF. Collagenous fibroma (desmoplastic fibroblastoma): a clinicopathologic analysis of 63 cases of a distinctive soft tissue lesion with stellate-shaped fibroblasts. Hum Pathol. 1998; 29:676–682.
8. Robbin MR, Murphey MD, Temple HT, Kransdorf MJ, Choi JJ. Imaging of musculoskeletal fibromatosis. Radiographics. 2001; 21:585–600.
9. Dinauer PA, Brixey CJ, Moncur JT, Fanburg-Smith JC, Murphey MD. Pathologic and MR imaging features of benign fibrous soft-tissue tumors in adults. Radiographics. 2007; 27:173–187.
10. Murphey MD, Gross TM, Rosenthal HG. From the archives of the AFIP. Musculoskeletal malignant fibrous histiocytoma: radiologic-pathologic correlation. Radiographics. 1994; 14:807–826. quiz 827-828.
11. Mahajan H, Kim EE, Wallace S, Abello R, Benjamin R, Evans HL. Magnetic resonance imaging of malignant fibrous histiocytoma. Magn Reson Imaging. 1989; 7:283–288.
12. Kransdorf MJ, Meis JM, Montgomery E. Elastofibroma: MR and CT appearance with radiologic-pathologic correlation. AJR Am J Roentgenol. 1992; 159:575–579.
13. Murphey MD, Smith WS, Smith SE, Kransdorf MJ, Temple MT. From the archives of the AFIP. Imaging of musculoskeletal neurogenic tumors: radiologic-pathologic correlation. Radiographics. 1999; 19:1253–1280.
14. Llauger J, Palmer J, Monill JM, Franquet T, Bagué S, Rosón N. MR imaging of benign soft-tissue masses of the foot and ankle. Radiographics. 1998; 18:1481–1498.
15. Hassell DS, Bancroft LW, Kransdorf MJ, et al. Imaging appearance of diffuse neurofibroma. AJR Am J Roentgenol. 2008; 190:582–588.
16. Llauger J, Palmer J, Roson N, Cremades R, Baque S. Pigmented villonodular synovitis and giant cell tumors of the tendon sheath: radiologic and pathologic features. AJR Am J Roentgenol. 1999; 172:1087–1091.
17. Sheldon PJ, Forrester DM, Learch TJ. Imaging of intraarticular masses. Radiographics. 2005; 25:105–119.
18. Murphey MD, Vidal JA, Fanburg-Smith JC, Gajewski DA. Imaging of synovial chondromatosis with radiologic-pathologic correlation. Radiographics. 2007; 27:1465–1488.
19. Kransdorf MJ, Meis JM, Jelinek JS. Myositis ossificans: MR appearance with radiologic-pathologic correlation. AJR Am J Roentgenol. 1991; 157:1243–1248.
20. Parikh J, Hyare H, Saifuddin A. The imaging features of posttraumatic myositis ossificans, with emphasis on MRI. Clin Radiol. 2002; 57:1058–1066.
21. De Beuckeleer LH, De Schepper AM, Neetens I. Magnetic resonance imaging of pilomatricoma. Eur Radiol. 1996; 6:72–75.
22. Lee SH, Suh JS, Lim BI, Yang WI, Shin KH. Intravascular papillary endothelial hyperplasia of the extremities: MR imaging findings with pathologic correlation. Eur Radiol. 2004; 14:822–826.
23. Clifford PD, Temple HT, Jorda M, Marecos E. Intravascular papillary endothelial hyperplasia (Masson's tumor) presenting as a triceps mass. Skeletal Radiol. 2004; 33:421–425.
24. Murphey MD, Fairbairn KJ, Parman LM, Baxter KG, Parsa MB, Smith WS. From the archives of the AFIP. Musculoskeletal angiomatous lesions: radiologic-pathologic correlation. Radiographics. 1995; 15:893–917.
25. Vilanova JC, Barcelo J, Smirniotopoulos JG, et al. Hemangioma from head to toe: MR imaging with pathologic correlation. Radiographics. 2004; 24:367–385.
26. Jones BC, Sundaram M, Kransdorf MJ. Synovial sarcoma: MR imaging findings in 34 patients. AJR Am J Roentgenol. 1993; 161:827–830.
27. Nakanishi K, Araki N, Yoshikawa H, Hashimoto T, Nakamura H. Alveolar soft part sarcoma. Eur Radiol. 1998; 8:813–816.
28. Hong SH, Chung HW, Choi JY, Koh YH, Choi JA, Kang HS. MRI findings of subcutaneous epidermal cysts: emphasis on the presence of rupture. AJR Am J Roentgenol. 2006; 186:961–966.
29. Ruzek KA, Wenger DE. The multiple faces of lymphoma of the musculoskeletal system. Skeletal Radiol. 2004; 33:1–8.
30. ter Braak BP, Guit GL, Bloem JL. Case 111: Soft-tissue lymphoma. Radiology. 2007; 243:293–296.
31. Boukobza M, Mazel C, Touboul E. Primary vertebral and spinal epidural non-Hodgkin's lymphoma with spinal cord compression. Neuroradiology. 1996; 38:333–337.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download