Abstract
Hypertensive encephalopathy and basal ganglia intracerebral hemorrhage (ICH) are a medical emergency caused by a sudden elevation of systemic blood pressure. Although the relationship between hypertensive encephalopathy and large ICH has not been clarified yet, Cushing reflex in acute elevations of ICP due to large ICH may induce or aggravate hypertensive encephalopathy. We report a rare case of isolated hypertensive brainstem encephalopathy combined with hypertensive ICH.
Hypertensive encephalopathy is an acute neurological syndrome caused by a sudden increase of systemic blood pressure (1, 2). The imaging abnormalities are predominant in the parieto-occipital subcortical white matter. The clinical and radiological abnormalities are reversible after antihypertensive treatment (3). Therefore, hypertensive encephalopathy has been called posterior reversible encephalopathy syndrome (PRES). Brainstem was involved in 58% of hypertensive encephalopathy in one study (4) but isolated brainstem involvement of hypertensive encephalopathy has been rarely reported (5, 6, 7, 8, 9).
In this case, rare isolated hypertensive brainstem encephalopathy was occurred with intracerebral hemorrhage (ICH). ICH is more common acute neurologic complication of systemic hypertension and may be an indicator of hypertensive attack in hypertensive encephalopathy. On the other hand, acute elevations of intracranial pressure (ICP) due to ICH can cause a further increase of systemic hypertension and an increased intracerebral capillary pressure and permeability (10, 11).
A 33-year-old man was transferred to our hospital. The patient presented with the initial symptoms including an altered mental status and a right side motor weakness. He was diagnosed with a left basal ganglia hemorrhage one day before he was transferred to our hospital. The patient had a history of hypertension; but there was no history of antihypertensive medication. The patient did not have a history of specific medication or disease.
On admission, the blood pressure was 250/200 mmHg, and after continuous infusion of antihypertensive drugs, the blood pressure decreased to 160/94 mmHg. The serum sodium level was normal (143 mmol/L), and the leukocyte count was elevated to 20,250 × 103/ul. The serum blood urea nitrogen and creatinine level was elevated to 24.5 mg/dL and 1.7 mg/dL, respectively. The laboratory findings associated with coagulation and other hematology were normal. The fluorescent antinuclear antibody (FNA), antidouble stranded DNA antibody, and cryoglobulin were all negative. Immunoglobulin levels were normal (IgG 1050 mg/dL, IgM 84.6 mg/dL, IgA 259 mg/dL) and the rheumatoid factor was normal (4 IU/mL). Serum glucose level was also in normal range.
Brain CT and MR imagings were performed on admission. The brain CT image showed a large hyperdense acute hematoma in the left basal ganglia. It is also noted as peripheral dark rim lesion on T2* gradient echo MR image (Fig. 1). On the T2-weighted and fluid attenuated inversion recovery (FLAIR) MR images, there was diffuse hyperintensity in the pons. It showed no enhancement after contrast injection. The hyperintensity mainly involved the central pons with relative sparing of the peripheral areas and some central fibers. There was no mass effect. This lesion showed no abnormal signal intensity on diffusion-weighted image (DWI) and slightly increased apparent diffusion coefficient (Fig. 1). There was no T2 hyperintense lesion in both cerebral and cerebellar hemispheres.
On six days after admission and the start of antihypertensive therapy, the blood pressure is much decreased (123/77 mmHg~148/90 mmHg) and the previous pontine hyperintensity was much improved on the follow-up T2WI and FLAIR (Fig. 2). A second MRI follow-up was obtained at three weeks and showed complete resolution of the pontine hyperintensity on T2WI and FLAIR (Fig. 2). The blood pressure was completely normalized (120/80 mmHg).
Kidney biopsy was performed to rule out of renal hypertension. The result was hypertensive nephrosclerosis which suggested that the cause of hypertension was not the renal origin. 24 hour urine aldosterone, metanephrine, normetanephrine levels were normal. There was no other evidence of secondary hypertension, either.
Typical hypertensive encephalopathy predominantly involves the posterior circulation territory including the parieto-occipital lobes. The MRI findings are bilateral diffuse or patchy hyperintensities on T2-weighted and FLAIR images without abnormality on DWI. In addition, high blood pressure at the initial presentation and rapid improvement after antihypertensive treatment would suggest this diagnosis.
Unusual locations, such as frontal lobe, basal ganglia, thalamus, brainstem, cerebellum, etc., have been reported (2, 3, 12). However, these lesions are usually accompanied by extensive parieto-occipital lesions (2). Like this case, isolated brainstem involvement without parieto-occipital lesion is rare (5, 6, 7, 8, 9).
Pathophysiology of hypertensive encephalopathy is associated with reversible vasogenic edema (13). A rapid increase in blood pressure leads to loss of cerebrovascular autoregulation and results in dilatation of the cerebral arterioles and breakdown of the blood-brain barrier. This leads to increased permeability and extravasation, and results in vasogenic edema (1, 2, 3, 12, 14).
Recently, two hypotheses were presented about isolated hypertensive brainstem encephalopathy. The brainstem may play a role of buffer to protecting distal parieto-occipital lobes in vertebrobasilar arterial supplying. Another one is fetal type of posterior cerebral artery with well developed posterior communicating artery, which may have well developed sympathetic innervation like anterior circulation (15, 16). However, well develop posterior communicating artery was not noted in this case.
In this case, typical hypertensive ICH was developed in company with hypertensive encephalopathy in pons. Hypertension is the most common cause of primary ICH and account for 60-70% of all ICH (17). In this case, large ICH in basal ganglia is one of the typical locations for hypertensive ICH and support the diagnosis of hypertensive encephalopathy in the pons. The abnormal signal intensity in the pons showed dramatic improvement after initiation of the antihypertensive treatment, which further supports the diagnosis (Fig. 2).
On the other hand, Cushing reflex in acute elevations of ICP due to large ICH may induce or aggravate hypertensive encephalopathy. Acute elevation of ICP also induces an increase in capillary pressure and permeability as well as a further increase in systemic blood pressure. It was identified by US neurosurgeon Henry Williams Cushing in 1901 and is called Cushing reflex (10, 11). However, there was no diagnostic evidence of ICP elevation in symptom or image finding in this case, such as bradycardia, abnormal respiratory pattern, cerebral herniation or empty sella findings, etc.
The differential diagnoses of hypertensive brainstem encephalopathy include osmotic myelinolysis, brainstem glioma, ischemic infarction and autoimmune vasculitis (5, 9, 12). Although the osmotic myelinolysis can be rarely occurred in normal sodium level, electrolyte imbalance is an important clue for diagnosis in the central osmotic myelinolysis (18, 19). The patient in this case was normal serum sodium level. Brainstem glioma is associated with mass effect and contrast enhancement. In this case, there were no such findings. An ischemic infarction could be differentiated by no hyperintensity on DWI and the laboratory findings related to autoimmune vasculitis were normal. Above all, the rapid improvement in MR image findings in response to the antihypertensive therapy supply the strong and pathognomonic diagnostic clue of hypertensive encephalopathy.
This case is a report about isolated hypertensive brainstem encephalopathy combined with large hypertensive ICH. The effect of large ICH to hypertensive brainstem encephalopathy must be studied in future.
Figures and Tables
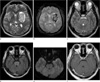 | Fig. 1MR images at the time of admission. (a) Transverse T2-weighted and (b) T2*-gradient echo MR images at the basal ganglia show a large acute stage hemorrhage in the left basal ganglia with mild perilesional edema. Another small hemorrhage is noted in the right basal ganglia. There is no abnormal signal intensity in the parieto-occipital areas on the T2-weighted image. (c) Transverse T2-weighted MR and (d) FLAIR images at the pons show diffuse hyperintensity in the pons with sparing of the peripheral areas. (e) This lesion shows no abnormal signal intensity on diffusion-weighted image. (f) There is no enhancement on contrast enhanced T1-weighted image. |
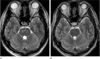 | Fig. 2Follow-up MR images on 6 days and 3 weeks later. (a) Transverse T2-weighted MR image on 6 days later shows an improved hyperintense lesion in pons but there is small residual patchy lesion. (b) Transverse T2-weighted MR image on 3 weeks later shows a complete improvement of pontine hyperintensity. |
References
1. Morello F, Marino A, Cigolini M, Cappellari F. Hypertensive brain stem encephalopathy: clinical silent massive edema of the pons. Neurol Sci. 2001; 22:317–320.
2. de Seze J, Mastain B, Stojkovic T, et al. Unusual MR findings of the brain stem in arterial hypertension. AJNR Am J Neuroradiol. 2000; 21:391–394.
3. Hinchey J, Chaves C, Appignani B, et al. A reversible posterior leukoencephalopathy syndrome. N Engl J Med. 1996; 334:494–500.
4. Bhagavati S, Chum F, Choi J. Hypertensive encephalopathy presenting with isolated brainstem and cerebellar edema. J Neuroimaging. 2008; 18:454–456.
5. Chang GY, Keane JR. Hypertensive brainstem encephalopathy: three cases presenting with severe brainstem edema. Neurology. 1999; 53:652–654.
6. Chang GY, Keane JR. Hypertensive brain stem encephalopathy. AJNR Am J Neuroradiol. 2000; 21(7):1366.
7. Thambisetty M, Biousse V, Newman NJ. Hypertensive brainstem encephalopathy: clinical and radiographic features. J Neurol Sci. 2003; 208:93–99.
8. Kang BW, Bae YJ, Cheon WH, Park SP, Suh CK. Two cases of hypertensive brainstem encephalopathy. J Korean Neurol Assoc. 2003; 21:535–538.
9. Gamanagatti S, Subramanian S. Hypertensive encephalopathy: isolated pons involvement mimicking central pontine myelinolysis. Korean J Radiol. 2006; 7:218–219.
10. Jones JV. Differentiation and investigation of primary versus secondary hypertension (Cushing reflex). Am J Cardiol. 1989; 63:10C–13C.
11. Fodstad H, Kelly PJ, Buchfelder M. History of the Cushing reflex. Neurosurgery. 2006; 59:1132–1137.
12. Casey SO, Truwit CL. Pontine reversible edema: a newly recognized imaging variant of hypertensive encephalopathy. AJNR Am J Neuroradiol. 2000; 21:243–245.
13. Kumai Y, Kazunori T, Kenichiro F, Setsuro I. Hypertensive encephalopathy extending into the whole brainstem and deep structures. Hypertens Res. 2002; 25:797–800.
14. Ay H, Buonanno FS, Schaefer PW, et al. Posterior leukoencephalopathy without severe hypertension: utility of diffusionweighted MRI. Neurology. 1998; 51:1369–1376.
15. Doi Y, Kimura F, Fujuyama T, et al. Hypertensive brainstem encephalopathy without parieto-occipital lesion-two case reports. Neurol Med Chir (Tokyo). 2006; 46:75–77.
16. Lee S, Cho BK, Kim H. Hypertensive encephalopathy with reversible brainstem edema. J Korean Neurosurg Soc. 2013; 54:139–141.
17. Mccormick WF, Rosenfield DB. Massive brain hemorrhage: a review of 144 cases and an examination of their causes. Stroke. 1973; 4:946–954.
18. de Morais BS, Carneiro FS, Araújo Rde M, Araújo GF, de Oliveira RB. Central pontine myelinolysis after liver transplantation: is sodium the only villain? Case report. Rev Bras Anestesiol. 2009; 59:344–349.
19. Lampl C, Yazdi K. Central pontine myelinolysis. Eur Neurol. 2002; 47:3–10.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download