Abstract
Purpose
To evaluate the relationship between superior labral dimension of the glenohumeral joint on direct MRA and presence of SLAP lesion.
Materials and Methods
IRB approval was obtained and informed consent was waived for this retrospective study. Direct MRA studies of the shoulder in 296 patients (300 shoulders) with arthroscopic surgery were analyzed by two radiologists blinded to the arthroscopic results, which were used as gold standard. One of the radiologists reviewed the images twice (session 1 and 2) for the evaluation of intra-observer variability. Transverse and longitudinal dimensions of superior labrum on coronal T1-weighted images were measured as base and height of the inverted triangular-shaped superior labrum and compared between patients with SLAP lesions vs. non-SLAP patients. Presence of meniscoid labrum was noted. Statistical analysis was done using unpaired t-test.
Results
Among 279 patients (283 shoulders), 122 patients (43.1%) had SLAP lesions. The mean base/height of superior labrum in SLAP and non-SLAP patients measured on T1-weighted MR image were 8.8 mm / 5.2 mm, 8.5 mm / 4.9 mm for reader 1; 8.2 mm / 4.9 mm, 8.1 mm / 4.5 mm for session 1 of reader 2; 8.0 mm / 4.8 mm, 7.6 mm / 4.3 mm for session 2 of reader 2. In SLAP group, the mean labral height was larger than non-SLAP group with statistically significant difference (p<0.05). Fifteen patients (5.3%) had meniscoid labrum according to operation records.
The glenoid labrum of the shoulder is a fibrous rim that surrounds and deepens the glenoid fossa and attaches confluently with the glenohumeral ligaments and the long head of biceps tendon to the peripheral glenoid. It has variable shape, size, and thickness, but along the superior part tends to be meniscus-like with mobile attachment (1). Several different anatomic variations of the glenoid labrum have been identified. Variations of the superior labrum are thought to occur due to loose attachment of superior labrum to the glenoid and variable insertions of the long head of the biceps tendon (2). There have been some studies describing the "meniscoid labrum", which covers the articular surface of the glenoid excessively, and it is thought to be a normal anatomical variant that does not require repair (3).
In 1990, Snyder et al. introduced the term of SLAP (Superior Labrum Anterior to Posterior) lesions that occur at the superior glenoid labrum (4) with separation of the labrum from the glenoid extending from anterior to posterior aspect to include the insertion site of the long head of the biceps. There have been several studies about the relationship between anatomical variations of the superior labrum and SLAP lesion (5, 6, 7), but there have been no studies about the relationship between the meniscoid labrum and SLAP lesion. There have been a few reports mentioning the meniscoid labrum as a normal variant of the superior glenoid labrum (8, 9); however, there has been no exact description of the diagnostic criterion or incidence for meniscoid labrum, in contrast to other variants, such as the sublabral hole or Buford complex, which have been more extensively described, and incidence reported (8).
Therefore, in this study we aimed to measure the superior labral dimension quantitatively on MR arthrography and to evaluate its relationship with SLAP lesion.
IRB approval was obtained and informed consent was waived for this retrospective study.
Among 959 patients who underwent direct MR arthrography at our institution due to shoulder pain or instability from May 2003 to December 2007, 296 patients (300 shoulders) were included who received arthroscopic shoulder surgery after MR arthrography. The time delay between MR arthrography and arthroscopy was less than 3 months. Seventeen patients (seventeen shoulders) were excluded due to poor MR image quality (mostly due to motion artifact), leaving 279 patients (283 shoulders) for evaluation. The resulting group consisted of 117 men (119 shoulders) and 162 women (164 shoulders), mean age of 58 years (range: 19-84 years). Arthroscopy was performed by an experienced orthopedic shoulder surgeon. Presence of SLAP lesion and meniscoid labrum was verified by reviewing operation records of all patients; meniscoid labrum was diagnosed when a superior labrum showed more than double the height of normal labrum on arthroscopy by the surgeon. We hypothesized there may be a higher incidence of meniscoid labrum in patients with SLAP vs. those without SLAP.
MR imaging was performed on a 1.5-T MR system (Intera, Philips Medical Systems, Netherlands) with dedicated shoulder coil. All patients had MR arthrographic examination after injecting 10-15 ml of gadobutrol (Gadovist®, Bayer Schering Pharma, Berlin, Germany) / normal saline mixture (1:200, 20 ml) into glenohumeral joint space by fluoroscopic guidance via anterior approach.
Two-dimensional fast spin echo (FSE) images were obtained in axial, oblique coronal and sagittal planes. Oblique coronal sequences were obtained perpendicular to the glenohumeral joint and oblique sagittal sequences, parallel to the glenoid medially up to scapular Y view. Axial, oblique coronal and sagittal T1-weighted fat-suppressed images were obtained with the following parameters: repetition time = 450-600 ms, echo time = 10-20 ms, echo train length = 4, flip angle = 90 and number of signal averages = 3. Oblique coronal T1-weighted images (repetition time = 500-600 ms, echo time = 90 ms, echo train length = 3, flip angle = 90, number of signal averages = 3), coronal and sagittal T2-weighted images (repetition time = 2900-3500 ms, echo time = 90 ms, echo train length = 17, flip angle = 90 and number of signal averages = 3) were acquired. Sixteen slices was obtained resulting in a slice thickness of 3 mm. The field of view was 140×140 mm, imaging matrix was 256×256.
Image evaluation was performed by one experienced faculty musculoskeletal radiologist (reader 1, 10 years of experience) and a trainee (reader 2, third-year resident of radiology department) independently and blind to arthroscopic records. Measuring the dimension of the labrum was performed on oblique coronal T1-weighted images at the level where long head of biceps looks smallest and labrum largest near the insertion site of long head of biceps tendon (about twelve o'clock position). The level was decided by consensus of the two readers. At each level, transverse and longitudinal dimensions were obtained by measuring the base and height of inverted triangular-shaped labrum (Fig. 1). Measurement was performed after consensus training session between the two readers for about 20 cases. Reader 2 reviewed the images twice at one-month interval (session 1 and session 2) for the evaluation of intra-observer variability.
For the evaluation of association between the presence of SLAP lesion and labral dimension, unpaired t-test was performed. P-values less than 0.05 were considered to have statistical significance. Interand intra-observer variability was evaluated between 2 readers and between sessions 1 and 2 of reader 2, respectively, using intra-class coefficients. Statistical analysis was done with commercially available software (MedCalc, Mariakerke, Belgium).
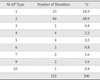
Among the 283 shoulders, 122 (43.1%) shoulders (men: n=66, women: n=56) were diagnosed to have SLAP lesion in the arthroscopic shoulder operation. Detailed description of the SLAP types is shown in Table 1. The mean base and height of the superior labrum in SLAP and non-SLAP patients are described in Table 2. Statistically, the mean height of the superior labrum was significantly larger in SLAP patients than in non-SLAP patients in all cases. The mean base of the superior labrum was not significantly different except in session 2 of reader 2. Fifteen patients were reported to have meniscoid labrum in the operation record and the mean labral height measured on T1-weighted MRI was 5.1 mm, 4.8 mm and 4.6 mm for reader 1, reader 2 session 1 and session 2, respectively (Table 3). Fourteen of fifteen (93%) shoulders with meniscoid labrum had SLAP lesions confirmed in the arthroscopic operation (Table 1); nine had type 2 SLAP lesions, four type 5, and one had type 1 SLAP lesion. Each case of normal superior labrum and meniscoid labrum with SLAP is shown in Fig. 2 and Fig. 3.
Intra-class correlation coefficient (ICC) was calculated to evaluate inter- and intra-observer variability of superior labral dimension measured on MRI. ICC was 0.76 between readers 1 and 2 on session 1, 0.68 between readers 1 and 2 on session 2 and 0.86 between sessions 1 and 2 of reader 2.
The superior glenoid labrum has been described extensively to be highly variable morphologically in many previous studies (10, 11, 12). The spectrum of normal variation of the superior labrum varies from firm attachment to complete absence of the superior labrum including conditions, such as sublabral recess, sublabral foramen or Buford complex (7, 13, 14). Meniscoid labrum is one of normal variations of the superior glenoid labrum. There have been few studies that described meniscoid labrum in the literature. Davidson et al. categorized the types of labrum that exist on the superior glenoid; they identified a bumper type of labrum in 18% of shoulders, meniscoid labrum in 38%, and a triangular labrum in 44% in a total of 191 consecutive patients who were prospectively evaluated arthroscopically (15). As such, although meniscoid labrum has been described in the previous literature, there are no suggested diagnostic criteria of the meniscoid labrum.
There are only few studies that focused on measuring labral dimension in the literature. Zanetti et al. evaluated MR arthrographic variability of the arthroscopically normal glenoid labrum both qualitatively and quantitatively (16). In the study, there was no significant relationship between quantitative dimensions and arthroscopically normal or abnormal status of the labrum.
There have been several studies about the relationship between SLAP lesions and anatomical variations of the anterosuperior labrum. Kanatli et al. suggested that anatomic variants of the anterosuperior labrum, such as sublabral foramen or Buford complex are associated with the development of SLAP lesions (5). But, there has been no previous study that evaluated the relationship between superior labral dimension or meniscoid labrum and SLAP lesion
In this study, overall prevalence of meniscoid labrum was 5.3% in the pre-operational patients. This was higher than the incidence reported in a previous study with 102 arthroscopically confirmed cases, which was 2.0% (9); in this previous study, there were two meniscoid-type labra, which were falsely diagnosed as SLAP on preoperative MR. However, in our study, there were actually SLAP lesions associated with 14 out of 15 meniscoid labra, suggesting a possible association between meniscoid labrum and the presence of SLAP lesions.
The mean measurement of heights measured by both radiologists showed statistically significant difference between SLAP and non-SLAP patients, which may suggest the possibility of larger dimension of the labrum as one of the predisposing conditions of SLAP lesion. However, the mean height of the superior labrum on MRI did not show significant difference between meniscoid and non - meniscoid labrum. This may be due to the small number of patients. On the other hand, there is a possibility that degenerative state of superior labrum with SLAP lesion may have caused it to appear ragged or redundant with scar tissue or adherent synovitis, leading to a "meniscoid" appearance, so the surgeon may have overcalled some as "meniscoid labrum" since there is no definitive diagnostic criterion for meniscoid labrum.
We think significant relationship between SLAP and labral dimension in this study possibly suggests that large labral dimension as such in meniscoid labrum can cause susceptible condition to SLAP. Large labrum may be exposed more on the glenohumeral articular surface and it may sustain more friction and be vulnerable to tear. But, on the other hand, we cannot exclude the possibility that labral change such as fraying or tear due to SLAP make labrum be seen larger than it really is. It can make the cause-and-effect relationship between SLAP and large labral dimension unclear.
This study has some limitations. First, there were some cases which showed vague boundary between labrum and long head of biceps tendon, which suggests that measured dimension may possibly represent part of the biceps-labral complex rather than pure labrum in some cases. Second, the relationship between larger superior labrum and SLAP was statistically significant in this study, but the actual difference in size of superior labrum was too small. Superior glenoid labrum is a small structure that is usually measured to be about 5 mm on MRI. The mean difference in height (0.3 mm/0.5 mm) between SLAP labrum and non-SLAP labrum was identical or less than the mean difference (0.5 mm/0.6 mm) of height between reader 1 and reader 2. Possibility of measurement error cannot be excluded although the intra-class coefficient was acceptable and this finding may limit the clinical value. Also, we cannot be certain whether the enlarged labrum is the predisposing condition for SLAP lesion or the result of SLAP with worn and ragged superior labrum appearing larger than in non-SLAP patients. Third, the study group was not a large size although larger than previous reports, including only small number of meniscoid labrum cases, so statistical analysis regarding the association between SLAP lesions and meniscoid labrum could not be performed although the incidence was high. Fourth, the gold standard in this study, i.e. arthroscopy, had its intrinsic limitations; there was no reference for diagnostic criterion of meniscoid labrum and there is a possibility that meniscoid labrum was overcalled.
In conclusion, in patients of SLAP lesion, the height of the superior glenoid labrum on oblique coronal image of MR arthrography was slightly larger than that of non-SLAP patients. A larger height of superior glenoid labrum may be associated with SLAP lesion although it may be either the cause or result of SLAP.
Figures and Tables
 | Fig. 1Measurement of superior labral dimension. (a) Superior labral dimension is measured on the plane where long head of biceps looks smallest and labrum largest twelve o'clock position. Transverse and longitudinal dimensions are obtained by measuring base (solid line) and height (dotted line) of inverted triangular shaped labrum. (b) On T1-weighted oblique coronal MRI, the base and height of the superior labrum are measured. |
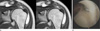 | Fig. 265-year-old female with rotator cuff tear. (a) Triangular shaped superior labrum attached to glenoid rim is seen on oblique coronal T1-weighted MR image. The height of the labrum is measured to be 6.4 mm. (b) There is neither meniscoid labrum nor SLAP on arthroscopy. |
 | Fig. 355-year-old female with rotator cuff tear and SLAP. (a) The height of labrum is measured to be 10.3 mm on oblique coronal T1-weighted MR image. It is considered to be larger than usual. (b) On arthroscopy, both meniscoid labrum (white arrow) and SLAP (black arrow) are detected. The superior labrum is covering upper quarter of the glenoid. |
Acknowledgement
This study was partially supported by grant no. 02-2012-051 from the SNUBH Research Fund and partially by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (NRF-2012R1A1A3010896).
The authors thank Division of Statistics in Medical Research Collaborating Center at Seoul National University Bundang Hospital for statistical analyses.
References
1. Bencardino JT, Beltran J, Rosenberg ZS, et al. Superior labrum anterior-posterior lesions: diagnosis with MR arthrography of the shoulder. Radiology. 2000; 214:267–271.
2. Vangsness CT Jr, Jorgenson SS, Watson T, Johnson DL. The origin of the long head of the biceps from the scapula and glenoid labrum. An anatomical study of 100 shoulders. J Bone Joint Surg Br. 1994; 76:951–954.
3. Lee SB, Harryman DT 2nd. Superior detachment of a glenoid labrum variant resembling an incomplete discoid meniscus in a wheelchair ambulator. Arthroscopy. 1997; 13:511–514.
4. Snyder SJ, Banas MP, Karzel RP. An analysis of 140 injuries to the superior glenoid labrum. J Shoulder Elbow Surg. 1995; 4:243–248.
5. Kanatli U, Ozturk BY, Bolukbasi S. Anatomical variations of the anterosuperior labrum: prevalence and association with type II superior labrum anterior-posterior (SLAP) lesions. J Shoulder Elbow Surg. 2010; 19:1199–1203.
6. Kreitner KF, Botchen K, Rude J, Bittinger F, Krummenauer F, Thelen M. Superior labrum and labral-bicipital complex: MR imaging with pathologic-anatomic and histologic correlation. AJR Am J Roentgenol. 1998; 170:599–605.
7. De Maeseneer M, Van Roy F, Lenchik L, et al. CT and MR arthrography of the normal and pathologic anterosuperior labrum and labral-bicipital complex. Radiographics. 2000; 20:S67–S81.
8. Tischer T, Vogt S, Kreuz PC, Imhoff AB. Arthroscopic anatomy, variants, and pathologic findings in shoulder instability. Arthroscopy. 2011; 27:1434–1443.
9. Connell DA, Potter HG, Wickiewicz TL, Altchek DW, Warren RF. Noncontrast magnetic resonance imaging of superior labral lesions 102 cases confirmed at arthroscopic surgery. Am J Sports Med. 1999; 27:208–213.
10. Cooper DE, Arnoczky SP, O'Brien SJ, Warren RF, DiCarlo E, Allen AA. Anatomy, histology, and vascularity of the glenoid labrum. An anatomical study. J Bone Joint Surg Am. 1992; 74:46–52.
11. Ilahi OA, Labbe MR, Cosculluela P. Variants of the anterosuperior glenoid labrum and associated pathology. Arthroscopy. 2002; 18:882–886.
12. Ide J, Maeda S, Takagi K. Normal variations of the glenohumeral ligament complex: an anatomic study for arthroscopic Bankart repair. Arthroscopy. 2004; 20:164–168.
13. Waldt S, Metz S, Burkart A, et al. Variants of the superior labrum and labro-bicipital complex: a comparative study of shoulder specimens using MR arthrography, multi-slice CT arthrography and anatomical dissection. Eur Radiol. 2006; 16:451–458.
14. Smith AM, McCauley TR, Jokl P. SLAP lesions of the glenoid labrum diagnosed with MR imaging. Skeletal Radiol. 1993; 22:507–510.
15. Davidson PA, Rivenburgh DW. Mobile superior glenoid labrum: a normal variant or pathologic condition? Am J Sports Med. 2004; 32:962–966.
16. Zanetti M, Carstensen T, Weishaupt D, Jost B, Hodler J. MR arthrographic variability of the arthroscopically normal glenoid labrum: qualitative and quantitative assessment. Eur Radiol. 2001; 11:559–566.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download