Abstract
Purpose
Intramedullary spinal lesions in the conus medullaris (CM), including tumors and vascular lesion, are rarely reported. We reported various MR features of intramedullary spinal cord lesions involving the CM including ependymoma, hemangioblastomas, dermoid cyst, ventriculus terminalis and spinal AVF and tried to discuss them for differential diagnosis.
Materials and Methods
Six patients (male: female = 4:2, mean age = 44.3 year old) were enrolled from the clinical database of our institute from 2004 to 2010 and their radiological images and clinical symptoms were reviewed retrospectively. All patients had taken initial and postoperative MRI with contrast enhancement using gadopentate dimeglumine (Gd-DTPA). These images were analyzed by tumor size, location, signal intensity relative to the spinal cord, vascular flow voids, syrinx or cyst, edema and enhancement pattern.
Results
Contrast enhancement was seen in all intramedullary masses. An eccentric enhancing nodule was noted in two hemangioblastomas and unusual peripheral rim enhancement with septation was seen in ventriculus terminalis. Patchy enhancement of the CM was observed in spinal arteriovenous fistula (AVF). Extensive cord edema adjacent to the intramedullary lesions was seen in four cases and syrinx was noted in three cases. Vascular signal voids were found in two hemangioblastomas and one spinal AVF.
Intramedullary spinal lesions in the conus medullaris (CM), including tumors, infection and vascular lesion, are rarely reported. Intramedullary spinal cord tumors account for 1% of all central nervous system tumors (1) and some infectious diseases in the CM were reported as case reports (2, 3, 4, 5, 6, 7, 8, 9, 10). Moreover some authors described syringomyelia of the CM due to spinal arteriovenous malformation (11). In a previous literature, 26.8% of 447 ependymomas were commonly located in the CM (12). Although they had a nonspecific radiologic appearance, Kim et al. (13) suggested some diagnostic clues of ependymoma were central location, diffuse enhancement, syringomyelia, hemorrhage and cap sign using MR imaging.
MR imaging is currently used as an imaging modality of choice in defining intramedullary spinal cord lesions (14, 15). MR imaging of the spinal cord tumor includes evaluation of tumor characteristics, the extent of cord involvement, enhancement pattern (14, 16).
Therefore we reported various MR features of intramedullary spinal cord involving the CM in six patients and tried to discuss them for differential diagnosis.
This retrospective study was approved by our institutional review board and the requirement for the informed consent was waived. Six patients (male: female = 4:2, mean age = 44.3 year old) were enrolled from the clinical database of our institute from 2004 to 2010 and their radiological images and clinical symptoms were reviewed retrospectively. Intramedul-lary CM tumor was defined as the primary spinal cord tumor involved only at CM. Pathology was confirmed in five patients after total surgical resection (Table 1).
All patients had taken initial and postoperative MRI with contrast enhancement using gadopentate dimeglumine (Gd-DTPA). Lumbar-sacral MR imaging was performed using 1.5-T system (Vision 1.5T and Avanto 1.5T; Simens medical systems, Erlangen, Germany). Axial and sagittal T1, T2-weighted fast spin-echo sequence and contrast enhanced T1-weighted images were taken in 1.5-T system (T1WI: TR/TE=560/9.8 msec; number of sections, 17; section thickness, 3 mm; field of view, 35 cm; matrix, 314 × 448; number of signals acquired, two; echo train length, 3; and voxel resolution, 1.1 × 0.8 × 3.0 mm, T2WI: TR/TE=3760/100 msec; number of sections, 17; section thickness, 3 mm; field of view, 35 cm; matrix, 338 × 512; number of signals acquired, four; echo train length, 3; and voxel resolution, ; 1.0 × 0.7 × 3.0 mm). These images were reviewed independently by two experienced neuroradiologists (S.J.A, T. S.C) and analyzed by the following parameters; tumor size, location, signal intensity relative to the spinal cord, vascular flow voids, syrinx or cyst, edema and enhancement pattern.
Six intramedullary spinal masses were located from the 12th level of the thoracic vertebra to the 2nd level of the lumbar vertebra and their size varied from 21 to 62 mm (Table 1). Three cystic masses were intermingled with the solid portion, which showed variable signal intensities on T1 weighted (T1WI) and T2-weighted images (T2WI) (Figs. 1, 3). Two purely cystic lesions showed the same signal as the cerebrospinal fluid (CSF) (Fig. 2). Contrast enhancement was seen in all intramedul-lary masses. An eccentric enhancing nodule was noted in two hemangioblastomas (Figs. 1a, 2b) and unusual peripheral rim enhancement with septation was seen in ventriculus terminalis (Fig. 2a). Patchy enhancement of the CM was observed in spinal arteriovenous fistula (AVF) (Fig. 1b). Extensive cord edema and hydrosyrinx was seen adjacent to the intramedullary lesions in three cases. Vascular signal voids were found in two hemangioblastomas and one spinal AVF which were confirmed as "dilated medullary vein" on spinal angiography. On the follow-up two cases (patient 1 and 2), which showed residual or recurrent contrast-MR imaging, four cases showed almost complete resolution after total surgical resection or embolization and enhancing lesions two years after surgery.
Spinal angiography was performed in three patients to evaluate preoperative embolization of two hemangioblastomas and one AVF and transarterial embolization was performed in the latter.
Five patients had frequent lower back pain, combined with leg pain in two and buttock pain in one. Urinary disturbance was seen in two patients. In spinal AVF, sudden-onset weakness in extremities and sensory change was noted. All patients improved their symptoms after operation or embolization.
We described six different cases of intramedullary spinal pathologies involving the CM, including ependymoma, hemangioblastomas, dermoid cyst, ventriculus terminalis and spinal AVF. Irrespective of tissue pathology, intramedullary lesions involving the CM had some MR imaging in common; 1) relatively larger mass, more than 20 mm with swelling of the CM, 2) contrast enhancement of the solid component, 3) central location on the CM except one hemangioblastoma. The unusual MR features in this study were syringomyelia and patch cord enhancement in spinal AVF, cystic wall enhancement with an internal septum in the ventriculus terminalis and peripheral rim enhancement of the dermoid cyst.
Tumor size of our five cases involving the CM was relatively large, more than 20 mm. Epstein et al. (17) suggested dominant motor symptoms were commonly associated with very large ependymomas and a poorer postoperative outcome secondary to the increased surgical risk. Although swelling of the CM was found, hydrosyrinx occurred in only three cases, two cases of which were combined with extensive cord edema and dilated medullary vein. The cause of syrinx and cord edema may be associated with intramedul-lary cord tumor as well as venous hypertension. While the syrinx was frequently reported in 9-50% of ependymoma (16, 18) and 40-81% of hemangioblastoma (19, 20, 21, 22), the syrinx was a rare pathology in case of spinal AVF. Srivatanakul et al. (11) reported that venous hypertension in the spinal cord was the trigger for the development of syringomyelia and showed disappearance of syrinx after transarterial embolization.
Typical MR imaging of spinal ependymomas usually show isointensity or slightly hypointensity on unenhanced T1-weighted images (15, 18, 23, 24). Ependymomas show hyperintencity in rare occasions, when the myxopapillary ependymoma is associated with hemorrhage or mucin production (18, 23). After gadolinium enhancement, lesions show homogenous, heterogenous or only rim enhancement (15, 18, 23, 24). About 20% of cases of ependymomas can show low signal intensity rim on T2-weighted images, which indicates the presence of hemosiderin deposit (23). Combined nontumoral cysts are common in about 78-84% ependymomas and incidence of tumoral cysts are about 4-50%, which is variable (16, 18, 23). Syringomyelia were variably associated in about 34-90% of ependymomas (18, 25). Our one case of ependymomas also showed cystic portion, which is also probably related to hemorrhage or necrosis.
Spinal hemangioblastomas shows hypointense to isointense on T1WI and isointense to hyperintense on T2WI (20, 26, 27, 28). On T2WI, hemangioblastomas can have intermixed focal flow voids (20, 26, 27, 28). Cyst formation or syringohydromyelia is very common (19, 20, 22, 26, 29) like our two cases. Our two cases of hemangioblastomas showed mostly cystic lesions with eccentric enhancing nodules which was compatible with typical MR findings of the previous studies (26, 28, 30). Both cases showed extensive cord edema and flow-void signal within the lesion.
The ventriculus terminalis is a round cystic cavity with smooth wall, no internal septa and no contrast enhancement of the cyst or its wall (31, 32, 33, 34, 35, 36, 37, 38). However, there was an internal septation and cystic wall enhancement in our case, which would lead to surgical resection because of misdiagnosis as spinal astrocytoma. Cystic wall enhancement was likely to be originated from venous hypertension or combined inflammatory change.
Spinal dermoid cysts showed usually hyperintensity on T1WI due to fatty secretion of sebaceous gland and cholesterol and usually hypointensity on T2WI but might be homogenous or heterogenous (39, 40). Spinal dermoid cysts did not usually enhance on postcontrast examination (3, 41). However our case showed heterogeneous intensity in T1 and T2WI as well as peripheral enhancement of the solid component (Fig. 3b). Enhancement of spinal dermoid cysts was a rare entity; only two cases were reported that was peripherally enhanced (42, 43).
Typical MR imaging of dural AVF show increased signal intensity on T2WI throughout the central area of the cord, particularly at CM and decreased signal intensity on T1WI. Prominent signal voids are frequently associated in subarachnoid space, which are the result of dilated vein. Spinal angiography provides the exact location and size of a lesion and information about feeding and draining vessels. Our case showed mostly cystic lesion with expansive cord edema and focal enhancement, probably due to chronic ischemic insult.
In conclusion, although this study had some limitation of heterogeneity and small numbers, intramedullary spinal pathologies of the CM have typical MR characteristics as well as some exceptional imaging features. In evaluation of intramedullary spinal lesions in the CM, it is necessary to consider these unusual MR findings and discriminate various pathologies with prudence and caution.
Figures and Tables
Fig. 1
Intramedullary hemangioblastoma (a) versus Spinal arteriovenous shunt (b).
a. T2-weighted sagittal image (left) shows solid mass of the conus medullaris with hydrosyrinx, extensive cord edema and dilated medullary veins. Contrast enhanced T1-weighted sagittal image (right) shows enhancement of two solid components. b. T2-weighted sagittal image (left) shows swelling and high signal intensity and atrophy of the conus medullaris with hydrosyrinx, dilated medullary vein and proximal cord edema. Contrast enhanced T1-weighted sagittal image (middle) shows focal enhancement of the conus medullaris, probably due to chronic ischemic insult from venous hypertension. On follow-up MR (right) cord edema and syrinx was disappeared after embolization.
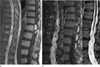
Fig. 2
Ventriculus terminalis (a) versus Intramedullary hemangioblastoma (b).
a. T2-weighted sagittal image (left) shows cystic mass in the conus medullaris without hydrosyrinx and cord edema. Contrast enhanced T1-weighted sagittal image (right) shows peripheral rim enhancement of the cyst with internal septum. b. T2-weighted sagittal image (left) shows cystic mass of the conus medullaris with extensive cord edema and dilated perimedullary vein. Contrast enhanced T1-weighted sagittal image (right) shows eccentric enhancement of the nodular component within the intratumoral cyst.
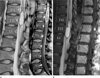
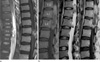
Fig. 3
Myxopapillary ependymoma (a) versus Dermoid cyst (b).
a. T2-weighted sagittal image (left) shows solid and cystic mass in the conus medullaris with extensive hydrosyrinx and cord edema. Contrast enhanced T1-weighted sagittal image (right) shows enhancement of the solid component. b. T1-weighted sagittal image (left) shows heterogeneous signal intensity within the intratumoral cyst and T2-weighted sagittal image (middle) shows solid and cystic mass of the conus medullaris without hydrosyrinx. Contrast enhanced T1-weighted sagittal image (right) shows rim enhancement of the solid component.
Acknowledgement
This study was supported by a faculty research grant of Yonsei University College of Medicine for 2013 (6-2013-0070).
References
1. Admiraal P, Hazenberg GJ, Algra PR, Kamphorst W, Wolbers JG. Spinal subarachnoid hemorrhage due to a filum terminale ependymoma. Clin Neurol Neurosurg. 1992; 94:69–72.
2. Hassan MF, Mohamed MB, Kalsi P, Sinar EJ, Bradey N. Intramedullary pyogenic abscess in the conus medullaris. Br J Neurosurg. 2012; 26:118–119.
3. Sharma M, Mally R, Velho V. Ruptured conus medullaris dermoid cyst with fat droplets in the central canal. Asian Spine J. 2013; 7:50–54.
4. Chen CY, Chen PH, Yao MS, Chu JS, Chan WP. MRI of hemangioblastoma in the conus medullaris. Comput Med Imaging Graph. 2008; 32:78–81.
5. Welling LC, Zanellato C, Tessari M, Mendes V, Figueiredo EG, Teixeira MJ. Hemangioblastoma of the conus medullaris. Br J Neurosurg. 2012; 26:296–297.
6. Shimosawa H, Matsumoto M, Yabe H, Mukai M, Toyama Y, Morioka H. Primary primitive neuroectodermal tumor of the conus medullaris in an elderly patient: a case report and review of the literature. Case Rep Oncol. 2011; 4:267–274.
7. Sanborn MR, Pramick M, Brooks J, Welch WC. Glioblastoma multiforme in the adult conus medullaris. J Clin Neurosci. 2011; 18:842–843.
8. Jaiswal AK, Jaiswal S, Gupta SK, Singh Gautam VK, Kumar S. Intramedullary tuberculoma of the conus. J Clin Neurosci. 2006; 13:870–872.
9. Kahilogullari G, Erdem A, Heper AO, Erden E. Intramedullary mature cystic teratoma of the conus medullaris. A case report. J Neurosurg Sci. 2006; 50:55–58.
10. Gallia GL, Burger PC, Suk I, et al. Concomitant conus medullaris ependymoma and filum terminale lipoma: case report. Neurosurgery. 2006; 58:E1214.
11. Srivatanakul K, Songsaeng D, Ozanne A, Toulgoat F, Lasjaunias P. Spinal arteriovenous malformation associated with syringomyelia. J Neurosurg Spine. 2009; 10:436–442.
12. Oh MC, Kim JM, Kaur G, et al. Prognosis by tumor location in adults with spinal ependymomas. J Neurosurg Spine. 2013; 18:226–235.
13. Kim DH, Kim JH, Choi SH, et al. Differentiation between intramedullary spinal ependymoma and astrocytoma: comparative MRI analysis. Clin Radiol. 2014; 69:29–35.
14. Goy AM, Pinto RS, Raghavendra BN, Epstein FJ, Kricheff II. Intramedullary spinal cord tumors: MR imaging, with emphasis on associated cysts. Radiology. 1986; 161:381–386.
15. Sun B, Wang C, Wang J, Liu A. MRI features of intramedullary spinal cord ependymomas. J Neuroimaging. 2003; 13:346–351.
16. Koeller KK, Rosenblum RS, Morrison AL. Neoplasms of the spinal cord and filum terminale: radiologic-pathologic correlation. Radiographics. 2000; 20:1721–1749.
17. Epstein FJ, Farmer JP, Freed D. Adult intramedullary spinal cord ependymomas: the result of surgery in 38 patients. J Neurosurg. 1993; 79:204–209.
18. Kahan H, Sklar EM, Post MJ, Bruce JH. MR characteristics of histopathologic subtypes of spinal ependymoma. AJNR Am J Neuroradiol. 1996; 17:143–150.
19. Browne TR, Adams RD, Roberson GH. Hemangioblastoma of the spinal cord. Review and report of five cases. Arch Neurol. 1976; 33:435–441.
20. Baker KB, Moran CJ, Wippold FJ 2nd, et al. MR imaging of spinal hemangioblastoma. AJR Am J Roentgenol. 2000; 174:377–382.
21. Park CH, Lee CH, Hyun SJ, Jahng TA, Kim HJ, Kim KJ. Surgical outcome of spinal cord hemangioblastomas. J Korean Neurosurg Soc. 2012; 52:221–227.
22. Murota T, Symon L. Surgical management of hemangioblastoma of the spinal cord: a report of 18 cases. Neurosurgery. 1989; 25:699–707.
23. Fine MJ, Kricheff II, Freed D, Epstein FJ. Spinal cord ependymomas: MR imaging features. Radiology. 1995; 197:655–658.
24. Yoshii S, Shimizu K, Ido K, Nakamura T. Ependymoma of the spinal cord and the cauda equina region. J Spinal Disord. 1999; 12:157–161.
25. Han IH, Kuh SU, Chin DK, Kim KS, Jin BH, Cho YE. Surgical treatment of primary spinal tumors in the conus medullaris. J Korean Neurosurg Soc. 2008; 44:72–77.
26. Lowe GM. Magnetic resonance imaging of intramedullary spinal cord tumors. J Neurooncol. 2000; 47:195–210.
27. Bostrom A, Hans FJ, Reinacher PC, et al. Intramedullary hemangioblastomas: timing of surgery, microsurgical technique and follow-up in 23 patients. Eur Spine J. 2008; 17:882–886.
28. Xu QW, Bao WM, Mao RL, Yang GY. Magnetic resonance imaging and microsurgical treatment of intramedullary hemangioblastoma of the spinal cord. Neurosurgery. 1994; 35:671–675.
29. Samii M, Klekamp J. Surgical results of 100 intramedullary tumors in relation to accompanying syringomyelia. Neurosurgery. 1994; 35:865–873.
30. Brisman JL, Borges LF, Ogilvy CS. Extramedullary hemangioblastoma of the conus medullaris. Acta Neurochir (Wien). 2000; 142:1059–1062.
31. Sigal R, Denys A, Halimi P, Shapeero L, Doyon D, Boudghene F. Ventriculus terminalis of the conus medullaris: MR imaging in four patients with congenital dilatation. AJNR Am J Neuroradiol. 1991; 12:733–737.
32. Matsubayashi R, Uchino A, Kato A, Kudo S, Sakai S, Murata S. Cystic dilatation of ventriculus terminalis in adults: MRI. Neuroradiology. 1998; 40:45–47.
33. Dullerud R, Server A, Berg-Johnsen J. MR imaging of ventriculus terminalis of the conus medullaris. A report of two operated patients and a review of the literature. Acta Radiol. 2003; 44:444–446.
34. Liccardo G, Ruggeri F, De Cerchio L, Floris R, Lunardi P. Fifth ventricle: an unusual cystic lesion of the conus medullaris. Spinal Cord. 2005; 43:381–384.
35. Brisman JL, Li M, Hamilton D, Mayberg MR, Newell DW. Cystic dilation of the conus ventriculus terminalis presenting as an acute cauda equina syndrome relieved by decompression and cyst drainage: case report. Neurosurgery. 2006; 58:E585.
36. Sansur CA, Sheehan JP, Sherman JH, Jane JA. Ventriculus terminalis causing back pain and urinary retention. Acta Neurochir (Wien). 2006; 148:919–920.
37. Ciappetta P, D'Urso PI, Luzzi S, Ingravallo G, Cimmino A, Resta L. Cystic dilation of the ventriculus terminalis in adults. J Neurosurg Spine. 2008; 8:92–99.
38. Dhillon RS, McKelvie PA, Wang YY, Han T, Murphy M. Cystic lesion of the ventriculus terminalis in an adult. J Clin Neurosci. 2010; 17:1601–1603.
39. van Aalst J, Hoekstra F, Beuls EA, et al. Intraspinal dermoid and epidermoid tumors: report of 18 cases and reappraisal of the literature. Pediatr Neurosurg. 2009; 45:281–290.
40. Graham DV, Tampieri D, Villemure JG. Intramedullary dermoid tumor diagnosed with the assistance of magnetic resonance imaging. Neurosurgery. 1988; 23:765–767.
41. Muthukumar N, Srisaravanan J. Intramedullary dermoid in a low lying conus tethered by a fatty filum - embryological implications. Acta Neurochir (Wien). 2007; 149:1173–1175.
42. Krishna KK, Agarwal PA, Agarwal SI, Jain MM. Dermoid of the conus medullaris. J Clin Neurosci. 2004; 11:796–797.
43. Patankar AP, Sheth JH. Dermoid cyst: a rare intramedullary inclusion cyst. Asian J Neurosurg. 2012; 7:81–83.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download