1. Fisher B, Anderson S, Bryant J, et al. Twenty-year follow-up of a randomized trial comparing total mastectomy, lumpectomy, and lumpectomy plus irradiation for the treatment of invasive breast cancer. N Engl J Med. 2002; 347:1233–1241.
2. Jacobson JA, Danforth DN, Cowan KH, et al. Ten-year results of a comparison of conservation with mastectomy in the treatment of stage I and II breast cancer. N Engl J Med. 1995; 332:907–911.
3. Morris AD, Morris RD, Wilson JF, et al. Breast-conserving therapy vs mastectomy in early-stage breast cancer: a meta-analysis of 10-year survival. Cancer J Sci Am. 1997; 3:6–12.
4. van Dongen JA, Voogd AC, Fentiman IS, et al. Long-term results of a randomized trial comparing breast-conserving therapy with mastectomy: European Organization for Research and Treatment of Cancer 10801 trial. J Natl Cancer Inst. 2000; 92:1143–1150.
5. Veronesi U, Cascinelli N, Mariani L, et al. Twenty-year follow-up of a randomized study comparing breast-conserving surgery with radical mastectomy for early breast cancer. N Engl J Med. 2002; 347:1227–1232.
6. Clarke M, Collins R, Darby S, et al. Effects of radiotherapy and of differences in the extent of surgery for early breast cancer on local recurrence and 15-year survival: an overview of the randomised trials. Lancet. 2005; 366:2087–2106.
7. Singletary SE. Surgical margins in patients with early-stage breast cancer treated with breast conservation therapy. Am J Surg. 2002; 184:383–393.
8. Bedrosian I, Mick R, Orel SG, et al. Changes in the surgical management of patients with breast carcinoma based on preoperative magnetic resonance imaging. Cancer. 2003; 98:468–473.
9. Berg WA, Gutierrez L, NessAiver MS, et al. Diagnostic accuracy of mammography, clinical examination, US, and MR imaging in preoperative assessment of breast cancer. Radiology. 2004; 233:830–849.
10. Camp ER, McAuliffe PF, Gilroy JS, et al. Minimizing local recurrence after breast conserving therapy using intraoperative shaved margins to determine pathologic tumor clearance. J Am Coll Surg. 2005; 201:855–861.
11. Chung A, Saouaf R, Scharre K, Phillips E. The impact of MRI on the treatment of DCIS. Am Surg. 2005; 71:705–710.
12. Del Frate C, Borghese L, Cedolini C, et al. Role of pre-surgical breast MRI in the management of invasive breast carcinoma. Breast. 2007; 16:469–481.
13. Fischer U, Kopka L, Grabbe E. Breast carcinoma: effect of preoperative contrast-enhanced MR imaging on the therapeutic approach. Radiology. 1999; 213:881–888.
14. Solin LJ, Orel SG, Hwang WT, Harris EE, Schnall MD. Relationship of breast magnetic resonance imaging to outcome after breast-conservation treatment with radiation for women with early-stage invasive breast carcinoma or ductal carcinoma in situ. J Clin Oncol. 2008; 26:386–391.
15. Tillman GF, Orel SG, Schnall MD, Schultz DJ, Tan JE, Solin LJ. Effect of breast magnetic resonance imaging on the clinical management of women with early-stage breast carcinoma. J Clin Oncol. 2002; 20:3413–3423.
16. Mann RM, Kuhl CK, Kinkel K, Boetes C. Breast MRI: guidelines from the European Society of Breast Imaging. Eur Radiol. 2008; 18:1307–1318.
17. Pettit K, Swatske ME, Gao F, et al. The impact of breast MRI on surgical decision-making: are patients at risk for mastectomy? J Surg Oncol. 2009; 100:553–558.
18. Fisher B, Anderson S, Redmond CK, Wolmark N, Wickerham DL, Cronin WM. Reanalysis and results after 12 years of follow-up in a randomized clinical trial comparing total mastectomy with lumpectomy with or without irradiation in the treatment of breast cancer. N Engl J Med. 1995; 333:1456–1461.
19. Radiology ACo. Breast Imaging Reoprting and Data System(BIRADS). 4th ed. Reston, VA: American College of Radiology;2003.
20. AJCC cancer staging manual. 6th ed. New York (NY): Springer;2002.
21. Anscher MS, Jones P, Prosnitz LR, et al. Local failure and margin status in early-stage breast carcinoma treated with conservation surgery and radiation therapy. Ann Surg. 1993; 218:22–28.
22. Leong C, Boyages J, Jayasinghe UW, et al. Effect of margins on ipsilateral breast tumor recurrence after breast conservation therapy for lymph node-negative breast carcinoma. Cancer. 2004; 100:1823–1832.
23. Silverstein MJ, Lagios MD, Groshen S, et al. The influence of margin width on local control of ductal carcinoma in situ of the breast. N Engl J Med. 1999; 340:1455–1461.
24. Carlson RW, McCormick B. Update: NCCN breast cancer Clinical Practice Guidelines. J Natl Compr Canc Netw. 2005; 3:Suppl 1. S7–S11.
25. Morrow M, Strom EA, Bassett LW, et al. Standard for breast conservation therapy in the management of invasive breast carcinoma. CA Cancer J Clin. 2002; 52:277–300.
26. Aziz D, Rawlinson E, Narod SA, et al. The role of reexcision for positive margins in optimizing local disease control after breast-conserving surgery for cancer. Breast J. 2006; 12:331–337.
27. Kuhl C, Kuhn W, Braun M, Schild H. Pre-operative staging of breast cancer with breast MRI: one step forward, two steps back? Breast. 2007; 16:Suppl 2. S34–S44.
28. O'Sullivan MJ, Li T, Freedman G, Morrow M. The effect of multiple reexcisions on the risk of local recurrence after breast conserving surgery. Ann Surg Oncol. 2007; 14:3133–3140.
29. Chagpar AB, Martin RC 2nd, Hagendoorn LJ, Chao C, McMasters KM. Lumpectomy margins are affected by tumor size and histologic subtype but not by biopsy technique. Am J Surg. 2004; 188:399–402.
30. Dillon MF, Hill AD, Quinn CM, McDermott EW, O'Higgins N. A pathologic assessment of adequate margin status in breast-conserving therapy. Ann Surg Oncol. 2006; 13:333–339.
31. Kurniawan ED, Wong MH, Windle I, et al. Predictors of surgical margin status in breast-conserving surgery within a breast screening program. Ann Surg Oncol. 2008; 15:2542–2549.
32. Miller AR, Brandao G, Prihoda TJ, Hill C, Cruz AB Jr, Yeh IT. Positive margins following surgical resection of breast carcinoma: analysis of pathologic correlates. J Surg Oncol. 2004; 86:134–140.
33. Smitt MC, Horst K. Association of clinical and pathologic variables with lumpectomy surgical margin status after preoperative diagnosis or excisional biopsy of invasive breast cancer. Ann Surg Oncol. 2007; 14:1040–1044.
34. Moore MM, Borossa G, Imbrie JZ, et al. Association of infiltrating lobular carcinoma with positive surgical margins after breast-conservation therapy. Ann Surg. 2000; 231:877–882.
35. Dzierzanowski M, Melville KA, Barnes PJ, MacIntosh RF, Caines JS, Porter GA. Ductal carcinoma in situ in core biopsies containing invasive breast cancer: correlation with extensive intraductal component and lumpectomy margins. J Surg Oncol. 2005; 90:71–76.
36. Mai KT, Chaudhuri M, Perkins DG, Mirsky D. Resection margin status in lumpectomy specimens for duct carcinoma of the breast: correlation with core biopsy and mammographic findings. J Surg Oncol. 2001; 78:189–193.
37. Hussien M, Lioe TF, Finnegan J, Spence RA. Surgical treatment for invasive lobular carcinoma of the breast. Breast. 2003; 12:23–35.
38. Raje D, Bollard R, Wilson A. Invasive lobular cancer of the breast--is breast conservation surgery a good option? Breast J. 2006; 12:574–575.
39. Mann RM, Loo CE, Wobbes T, et al. The impact of preoperative breast MRI on the re-excision rate in invasive lobular carcinoma of the breast. Breast Cancer Res Treat. 2010; 119:415–422.
40. Cabioglu N, Hunt KK, Sahin AA, et al. Role for intraoperative margin assessment in patients undergoing breast-conserving surgery. Ann Surg Oncol. 2007; 14:1458–1471.
41. Ramanah R, Pivot X, Sautiere JL, Maillet R, Riethmuller D. Predictors of re-excision for positive or close margins in breast-conservation therapy for pT1 tumors. Am J Surg. 2008; 195:770–774.
42. Onesti JK, Mangus BE, Helmer SD, Osland JS. Breast cancer tumor size: correlation between magnetic resonance imaging and pathology measurements. Am J Surg. 2008; 196:844–848. discussion 849-850.
43. Raza S, Vallejo M, Chikarmane SA, Birdwell RL. Pure ductal carcinoma in situ: a range of MRI features. AJR Am J Roentgenol. 2008; 191:689–699.
44. Clough KB, Lewis JS, Couturaud B, Fitoussi A, Nos C, Falcou MC. Oncoplastic techniques allow extensive resections for breast-conserving therapy of breast carcinomas. Ann Surg. 2003; 237:26–34.
45. Rainsbury RM. Surgery insight: oncoplastic breast-conserving reconstruction--indications, benefits, choices and outcomes. Nat Clin Pract Oncol. 2007; 4:657–664.
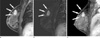

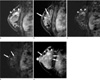





 PDF
PDF ePub
ePub Citation
Citation Print
Print






 XML Download
XML Download