Abstract
When cerebral cortical hyperintensities on diffusion-weighted image are seen in patients with suspected acute stroke accompanying seizure, it is necessary to differentiate whether they are caused by infarction or seizure-related change. We report a case of seizure-related cortical hypertensities in a patient with suspected acute infarction. With perfusion MR imaging, we could differentiate from acute infarction.
It has been reported that seizure-related hyperintense lesions can be demonstrated in the cerebral cortices on diffusion-weighted image (DWI). They are usually reversible but can become irreversible and permanent in severe or prolonged seizures (1, 2).
Acute infarction is the most common cause of the cerebral cortical hyperintensities on DWI. However, seizure-related change and encephalitis can also show cortical hyperintensities on DWI (1-3). Some patients with acute infarction show seizures in the clinical manifestations (4). Therefore, it is necessary to differentiate whether the DWI hyperintensities in the cerebral cortices are caused by infarction or seizure-related change. Sometimes, it is not easy to differentiate between acute infarction and seizure-related lesions with only conventional imaging findings. We report a case of seizure-related cortical hyperintensities in a patient with suspected acute infarction. With perfusion MR imaging, it was possible to differentiate from infarction.
A 45-year-old male patient was presented with tonic-clonic seizure and left hemiparesis, 1 hour 30 minutes ago. On the neurologic examinations at admission, his mentality was drowsy. He had decreased motor function of his upper and lower limbs as grade II on the left side and had mild to moderate dysarthria. The NIH stroke score was eight. On the past medical history, 3.5 years ago, he suffered hypertensive intracerebral hemorrhage (ICH) and fully recovered without any neurologic sequelae. He has been managed with oral antihypertensive medication. According to the stroke imaging protocol of our institution, MR examination was performed with a 1.5T scanner (Avanto, Siemens, Germany). On MR, there was tissue loss due to previous ICH in the right temporoparietal lobes. DWI showed cortical hyperintensities in the right temporoparietal lobes adjacent to the hemorrhagic tissue loss. The lesions showed slightly decreased signal intensities on apparent diffusion coefficient (ADC) map images. On fluid-attenuated inversion recovery (FLAIR) images, the lesions showed subtle hyperintensities. Dynamic susceptibility contrast MR perfusion images showed increased cerebral blood flow (CBF) and decreased mean transit time (MTT) of the lesions (Fig. 1). On contrast-enhanced MR angiography, there was no significant stenosis or occlusion in the neck and intracranial arteries. Electrocardiography was normal and cardiac echo was also normal without mild left ventricular dysfunction. There was no definite abnormality on electroencephalography. After the MR examination, his symptoms gradually improved. He fully recovered 24 hours later. Third day follow-up MR imaging showed complete resolution of the lesions. The patient was discharged the next day.
Treatment of thrombolytic agent can be considered when DWI hyperintensities are seen in the cerebral cortices in patients with suspected acute infarction. Decision for thrombolytic therapy should be cautious because it may cause hemorrhage as a complication, which can be potentially harmful to the patients. According to the European Cooperative Acute Stroke Study II (ECASS II), 17% of the patients who received thrombolytic treatment were finally proven not to have suffered strokes (5). In cases of cerebral cortical hyperintensities on DWI, not only acute infarction, but also seizure-related lesions, encephalitis, hypoxicischemic encephalopathy (HIE), and hypoglycemic encephalopathy should be included in the differential diagnosis (1-3, 6). Encephalitis can usually be diagnosed by cerebral spinal fluid (CSF) study. Considering symmetric distributions of the lesions and clinical history and blood glucose level of the patients, it is not difficult to diagnose HIE and hypoglycemic encephalopathy.
Seizures can be associated in patients with acute stroke (4). Patients with seizure can also be associated with focal neurologic deficits such as aphasia or hemiparesis, which can mimic stroke (7). Therefore, when DWI hyperintensities in the cerebral cortices are seen in patients with suspected stroke accompanying seizure, it is necessary to differentiate whether they are caused by infarction or seizure-related change. If they are caused by seizure-related change, thrombolytic treatment is not indicated. With only the conventional imaging findings, it is not easy to differentiate between acute infarction and seizure-related lesions.
In the present case, with perfusion MR imaging, we could differentiate seizure-related change from acute infarction in a patient with suspected stroke accompanying seizure. According to a report which was evaluated with single photon emission computed tomography and perfusion MR imaging in seizure patients, CBF and MTT of the lesions were increased and shortened, respectively in the ictal and postictal periods (8). Acute infarction usually shows decreased CBF and delayed MTT and time to peak (TTP), respectively (9).
In summary, in our patient with suspected acute infarction, seizure-related cortical hypertensities could be differentiated from infarction with perfusion MR imaging.
Figures and Tables
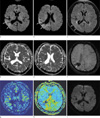
Fig. 1
A 45-year-old male patient with left hemiparesis and seizure.
Diffusion-weighted images (a, b) show hyperintense lesion in the right temporoparietal lobe cortices (arrows). On fluid-attenuated inversion recovery image (c), the lesion shows subtle hyperintensity (arrows). Apparent diffusion coefficient map images (d, e) show restricted water diffusion of the lesion (arrows). There is tissue loss due to old hemorrhage in the parietal lobe on susceptibility-weighted image (f) (arrow). Perfusion MR images show increased cerebral blood flow (g) and shortened mean transit time (h) in the corresponding area (arrows). On follow-up diffusion image 3 days later (i), the lesion has disappeared.
References
1. Cianfoni A, Caulo M, Cerase A, et al. Seizure-induced brain lesions: a wide spectrum of variably reversible MRI abnormalities. Eur J Radiol. 2013; 82:1964–1972.
2. Kim JA, Chung JI, Yoon PH, et al. Transient MR signal changes in patients with generalized tonicoclonic seizure or status epilepticus: periictal diffusion-weighted imaging. AJNR Am J Neuroradiol. 2001; 22:1149–1160.
3. Kiroğlu Y, Calli C, Yunten N, et al. Diffusion-weighted MR imaging of viral encephalitis. Neuroradiology. 2006; 48:875–880.
4. Reith J, Jørgensen HS, Nakayama H, Raaschou HO, Olsen TS. Seizures in acute stroke: predictors and prognostic significance. The Copenhagen Stroke Study. Stroke. 1997; 28:1585–1589.
5. The Multicenter Acute Stroke Trial-Europe Study Group. Thrombolytic therapy with streptokinase in acute ischemic stroke. N Engl J Med. 1996; 335:145–150.
6. Gutierrez LG, Rovira A, Portela LA, Leite Cda C, Lucato LT. CT and MR in non-neonatal hypoxic-ischemic encephalopathy: radiological findings with pathophysiological correlations. Neuroradiology. 2010; 52:949–976.
7. Masterson K, Vargas MI, Delavelle J. Postictal deficit mimicking stroke: role of perfusion CT. J Neuroradiol. 2009; 36:48–51.
8. Szabo K, Poepel A, Pohlmann-Eden B, et al. Diffusion-weighted and perfusion MRI demonstrates parenchymal changes in complex partial status epilepticus. Brain. 2005; 128:1369–1376.
9. Hochberg AR, Young GS. Cerebral perfusion imaging. Semin Neurol. 2012; 32:454–465.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download