Abstract
Purpose
The objective of this study was to analyze the brain volume according to the brain image of healthy adults in the 20s taken with different inversion time (TI).
Materials and Methods
Brain images of healthy adults in the 20 s were acquired using magnetization prepared rapid acquisition gradient echo (MPRAGE) pulse sequence with 1.5 mm thickness of pieces and four inversion times (1100 ms, 1000 ms, 900 ms, 800 ms). The acquired brain images were analyzed to measure the volume of white matter (WM), gray matter (GM), intracranial volume (ICV). The statistical difference according to brain volume and gender was analyzed for each TI.
Results
The brain volume calculated using Freesurfer was WM=486.52±48.64 cm3 and GM=646.86±57.12 cm3 in mean when adjusted by mean ICV=1278.94±154.92 cm3. Men's brain volume(WM, GM, ICV) was larger than women's brain volume. In the intrarater reliability test, all of the intraclass correlation coefficients were high (0.992 for WM, 0.988 for GM, and 0.997 for ICV). In the repeated measures analysis of variance, GM and ICV did not show a significant difference at each TI (GM p=0.143, ICV p=0.052), but WM showed a significant (p=0.001). In the linear structure relation analysis, all of the Pearson correlation coefficients were high.
Conclusion
WM, GM, and ICV indicated high reliability and solid linear structure relations, but WM showed significant differences at each TI. The brain volume of healthy adults in the 20s could be used in comparison with that of patients for reference purposes and to predict the structural change of brain. It would be needed to conduct additional studies to examine the contract, SNR, and lesion detection ability according to variable TI.
Figures and Tables
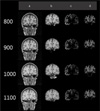 | Fig. 1The results of images from the 3D MRI using automatic segmentation software. Original image (a), brain mask image (b), that removed skull from the original image (c), white matter (d). (Numerical values are inversion time in milliseconds) |
 | Fig. 2The results of each subject is represented as mean volumes on the image acquisition conditions (white matter, gray matter, intracranial volume). |
 | Fig. 3Scatter plot matrix for correlation analysis of inversion times of brain volumes (white matter, gray matter, intracranial volume). |
Table 1
The Mean Volumes(Adjusted to Intracranial Volume) for the White Matter, Gray Matter and Intracranial Volume (means±SD) Depending on the Inversion Time

References
1. Takao H, Abe O, Ohtomo K. Computational analysis of cerebral cortex. Neuroradiology. 2010; 52:691–698.
2. Firbank MJ, Barber R, Burton EJ, O'brien JT. Validation of a fully automated hippocampal segmentation method on patients with dementia. Hum Brain Mapp. 2008; 29:1442–1449.
3. Klöppel S, Stonnington CM, Barnes J, et al. Accuracy of dementia diagnosis: a direct comparison between radiologists and a computerized method. Brain. 2008; 131:2969–2974.
4. Ashburner J, Friston KJ. Voxel-based morphometry-the methods. Neuroimage. 2000; 11:805–821.
5. Mechelli A, Cathy JP, Friston KJ, Ashburner J. Voxel-based morphometry of the human brain : methods and applications. Curr Med Imaging Rev. 2005; 1:105–113.
6. Khan AR, Wang L, Beg MF. FreeSurfer-initiated fullyautomated subsortical brain segmentation in MRI using large deformation diffeomorphic metric mapping. Neuroimage. 2008; 41:735–746.
7. Fischl B. Freesurfer. Neuroimage. 2012; 62:774–781.
8. Magnotta VA, Friedman L. FIRST BIRN. Measurement of signal to noise and contrast to noise in the fBIRN multicenter imaging study. J Digit Imaging. 2006; 19:140–147.
9. Friston KJ, Hoimes AP, Worsley KJ, Poline JP, Frith CD, Frackowiak RSJ. Statistical parametric maps in functional imaging: a general linear approach. Hum Brain Mapp. 1995; 2:189–210.
10. Hsu YY, Schuff N, Du AT, et al. Comparison of automated and manual MRI volumetry of hippocampus in normal aging and dementia. J Magn Reson Imaging. 2002; 16:305–310.
11. Seizas FL, Silveira AS. Anatomical brain MRI segmentation methods: volumetric assessment of the hippocampus. IWSSIP. 2010; 17:247–251.
12. Sigurdsson S, Aspelund T, Forsberg L, et al. Brain tissue volumes in the general population of the elderly: the AGES-Reykjavik study. Neuroimage. 2012; 59:3862–3870.
13. Giedd JN. The teen brian : insights from neuroimaging. J Adolesc Health. 2008; 42:335–343.
14. Ikram MA, Vrooman HA, Vernooij MW, et al. Brain tissue volumes in the general elderly population: the rotterdam scan study. Neurobiol Aging. 2008; 29:882–890.
15. Hashemi HR, Bradley WG, Lisanti CJ. MRI-The Basics. 3rd ed. philadelphia: Lippincott Williams & Wilkins;2010. 2:p. 173–183.
16. Hwang K, Velanova K, Luna B. Strengthening of top-down frontal cognitive control networks underlying the development of inhibitory control: a functional magnetic resonance imaging effective connectivity study. J Neurosci. 2010; 30:15535–15545.
17. Sowell ER, Thompson PM, Tessner KD, Toga AW. Mapping continued brain growth and gray matter density reduction in dorsal frontal cortex: inverse relationships during postadolescent brain maturation. J Neurosci. 2001; 21:8819–8829.
18. Gogtay N, Giedd JN, Lusk L, et al. Dynamic mapping of human cortical development during childhood through early adulthood. Proc Natl Acad Sci USA. 2004; 101:8174–8179.
19. Allen JS, Bruss J, Brown CK, Damasio H. Normal neuroanatomical variation due to age: the major loves and a parcellation of the temporal region. Neurobiol Aging. 2005; 26:1245–1260.
20. Fotenos AF, Snyder AZ, Girton LE, Morris JC, Buckner RL. Normative estimates of cross-sectional and longitudinal brain volume decline in aging and AD. Neurology. 2005; 64:1032–1039.
21. Ge Y, Crossman RI, Babb JS, Rabin ML, Mannon Lj, Kolson DL. Age-related total gray matter and white matter changes in normal adult brain. AJNR Am J Neuroradiol. 2002; 23:1327–1333.
22. Walhovd KB, Fjell AM, Reinvang I, et al. Effects of age on volumes of cortex, white matter and subcortical structures. Neurobiol Aging. 2005; 26:1261–1270.
23. Jernigan TL, Archibald SL, Notestine CF, et al. Effects of age on tissues and regions of the cerebrum and cerebellum. Neurobiol Aging. 2001; 22:581–594.
24. Resnick SM, Pham DL, Kraut MA, Zonderman AB, Davatzikos C. Longitudinal magnetic resonance imaging sutdies of older adults: a shrinking brain. J Neurosci. 2003; 23:3295–3301.
25. Taki Y, Goto R, Ecans A, et al. Voxel-based morphometry of human brain with age and cerebrovascular risk factors. Neurobiol Aging. 2004; 25:455–463.
26. Good CD, Johnsrude IS, Ashburner J, et al. A voxel-based morphometric study of ageing in 465 normal adult human brains. Neuroimage. 2001; 14:21–36.
27. Xu J, Kobayashi S, Yamaguchi S, Iijima K, Okada K, Yamashita K. Gender effects on age-related changes in brain structure. AJNR Am J Neuroradiol. 2000; 21:112–118.
28. Greenberg DL, Messer DF, Payne ME, et al. Aging, gender, and the elderly adult brain: an examination of analytical strategies. Neurobiol Aging. 2008; 29:290–302.
29. Takahashi M, Uematsu H, Hatabu H. MR imaging at high magnetic fields. Eur J Radiol. 2003; 46:45–52.
30. Lee JS, Lee DS, Kim JS, et al. Development of korean standard brain templates. J Korean Med Sci. 2005; 20:483–488.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download