Abstract
Materials and Methods
We collected information of the breast cancer patients who underwent the test for BRCA gene mutation as well as preoperative breast MRI from January 2007 to December 2010. A total of 185 patients were enrolled; 33 of these patients had BRCA mutations and 152 patients did not. Among them, a total of 231 breast cancers were detected. Images of the 47 breast cancers with BRCA mutation and of the 184 breast cancers without mutations were evaluated to compare the morphologic and enhancement features on MRI.
Results
With MR imaging, there were no significant difference in morphologic characteristic between two groups. However, enhancement pattern in the group with BRCA mutation were more likely to have persistent enhancement (p < 0.233), and LN metastasis was more common in breast cancers without BRCA mutation. Breast cancers with BRCA 2 mutation tend to show more persistent enhancement pattern than BRCA 1 mutation.
Breast cancer is the most prevalent malignancy among women, occurring in approximately one in eight women in Western countries (1). The BRCA1 and BRCA2 genes contribute 5~10% in genetic breast cancer and women with these mutations have a 60~80% risk of developing breast cancer by the age of 70 (2). To reduce this risk, close surveillance for the earlier detection of breast cancer at the most favorable stage is necessary.
The strategy for the surveillance of high-risk women should differ from that of the general population. Screening of high-risk women should begin at a younger age, and clinical breast examinations and annual mammography should begin at 25-35 years of age (3, 4). Findings of several studies indicate that contrast-enhanced MRI is useful for screening high-risk women (5, 6). In addition, the American Cancer Society guidelines recommend an annual MRI screening as an adjunct to mammography for BRCA mutation breast cancers due to the high sensitivity level achieved with MRI. However, the sensitivity levels achieved with MRI have a wide range (71~92%) (7-10) which means that a considerable number of breast cancers may go undetected by MRI perhaps because of the apparently benign features seen on breast MRI.
The purposes of our study are to retrospectively review the imaging features and characteristics of MRI of breast cancers occurring in Korean women with BRCA mutations. And to compare the MR imaging findings of breast cancers in BRCA mutations and without BRCA mutations as well as comparing the MRI findings in breast cancers with BRCA1 carriers and BRCA2 carriers.
This retrospective study was approved by our institutional review board, and the requirement to obtain informed consent was waived. From January 2007 to December 2010, a total 185 patients who underwent preoperative breast MRI and a gene mutation test for monitoring of their BRCA mutation, were included in the study population. In our institution, we usually recommend monitoring of BRCA mutations in breast cancer patients younger than 35 years of age as this is a generally accepted definition of young breast cancer patients in Korea and also breast cancer patients with a strong family history of more than two family members having a history of breast cancer.
Among these 185 patients, 33 had a proven mutation in one of the breast cancer susceptibility genes (BRCA1 or BRCA2), while 152 patients did not. A total of 47 breast cancers were detected in the BRCA mutation group patients, and 184 breast cancers were detected in the breast cancer patient group without BRCA mutations. For the BRCA1 carriers and the BRCA2 carriers, a total of 15 breast cancers and 32 breast cancers were included in the study, respectively.
All patients were scanned on a 1.5 T scanner (Magnetom Avanto, Siemens Medical Solutions, Erlangen, Germany) with a bilateral breast array coil (Siemens Medical Solutions). The standard MRI protocol included the following pulse sequences: 1) an axial two-dimensional T2 weighted short tau inversion recovery (STIR) turbo spin-echo pulse sequence (repetition time/echo time/time interval (TR/TE/TI), 6700/74/150 ms; field of view (FOV) 300 × 300 mm; matrix = 448 × 448; slice thickness 5 mm); 2) pre- and post-contrast-enhanced fat-saturated axial three-dimensional T1 weighted fast low angle shot volume-interpolated breath-hold examination (FLASH VIBE) pulse sequences (TR/TE, 5.2/2.4 ms; FOV 340 × 340 mm; matrix = 384 × 384; slice thickness 0.9 mm); and 3) an axial three-dimensional delayed contrast-enhanced turbo spin-echo pulse sequence (TR/TE, 767/12 ms; FOV 350 × 350 mm; matrix = 768 × 768; slice thickness, 5 mm) used for the evaluation of the supraclavicular and axillary lymph nodes. The six dynamic sequences were performed before and after contrast medium injection. The contrast medium (0.2 ml kg-1 body weight; Magnevist; Schering, Berlin, Germany) was injected using an MR-compatible power injector (Spectris; Medrad, Pittsburgh, PA, USA) with a flow of 1 ml s-1 followed by a 20-ml saline flush. Post-processing manipulation included the production of standard subtraction, reverse subtraction, and maximum-intensity-projection images.
MRI scans were retrospectively reviewed by two radiologists with 5 and 13 years, respectively, of clinical experience in breast radiology. Each radiologist was blinded to the other radiologist's readings during the initial review. When there was a discrepancy, the two radiologists reviewed the cases together and reached a consensus.
The MRI findings and the corresponding kinetic curve assessments were described using the terminology in the ACR BI-RADS MRI lexicon. For mass lesions, the morphologic analysis included lesion margin, shape, and enhancement pattern. Margins were described as smooth, indistinct, uneven or spiculated. Lesion shape was classified as round, oval, lobulated or irregular. The internal enhancement pattern for mass was classified as homogeneous, heterogeneous or rim enhancement. For non-mass lesions, the distribution and enhancement pattern were evaluated and distribution was described as focus, linear, segmental or diffuse. The enhancement pattern was classified as homogeneous, heterogeneous or clumped. Lesion enhancement kinetic curve analysis was also described as Type 1 showing persistent enhancement, Type 2 showing plateau enhancement, and Type 3 with peak early enhancement followed by delayed washout. The presence of lymph node metastasis and the size of the each tumor were also evaluated for comparison between two groups.
We also included the comparison of the morphologic characteristics and enhancement patterns of BRCA1 carrier and BRCA2 carrier breast cancers. Imaging features were also recorded according to the ACR BIRADS MRI lexicon, as described above.
The difference in tumor characteristics between the non-BRCA mutation group and the BRCA group was analyzed using the χ2 test or Fisher's exact test for the categorical variables and the Mann-Whitney test for the continuous variables. The p-value was less than 0.01 in our study.
The mean patient age was 39.2-years (age range, 19~71 years), and the mean age of the BRCA carriers and the non-BRCA carriers was 36.1 years (range, 19~71) and 39.8 years (range, 23~59), respectively.
A total of 47 breast cancers with BRCA mutation and 184 breast cancers without BRCA mutation were included in our study population. The dominant pathologic types of tumors are shown in Fig. 1. Invasive ductal carcinoma was the most common pathologic type in breast cancers both with (n = 36, 76.6%) and without BRCA mutation (n = 125, 67.9%). The mean pathologic tumor size was 3.09±2.2 cm for the BRCA carrier group and 3.29±2.25 cm for the non-BRCA carrier group. No significant statistical difference was found in the age (p = 0.074) or pathologic tumor size (p = 0.504) between the two groups.
Out of a total 231 lesions, 186 were mass lesions and 45 were non-mass lesions. Of 47 lesions in the BRCA carrier group, there were 43 mass lesions (91.4%) and 4 non-mass lesions (8.6%). Of the 184 lesions in the non-BRCA carrier group, there were 143 masses (77.7%) and 41 non-mass lesions (22.3%). There was no significant statistical difference in the number of mass and non-mass tumor lesions between the BRCA carrier and the non-BRCA carrier groups.
Although the dominant morphologic MRI characteristics (Table 1) of mass lesions were their irregular shape (69.8%), indistinct margin (62.8%) and heterogenous enhancement (72.1%) in the BRCA carrier group, they tend to show smoother margins compared to non-BRCA carriers. For non-mass lesions, segmental distribution (75%) and heterogeneous enhancement (100%) were the dominant MR imaging features seen in the BRCA carrier group although there were no significant statistical difference between BRCA and non-BRCA in non-mass lesions (Table 2).
Most cancers showed a Type 3 enhancement curve type with a wash-out pattern in both the BRCA carrier and non-BRCA carrier groups, and no statistical significant difference was found between these two groups regarding the enhancement kinetics (Table 3). However, BRCA carrier breast cancers have a tendency to show Type 1 persistent enhancement curve (p = 0.233) compared with non-BRCA carrier breast cancers, and which is more commonly associated with benign lesions (Fig. 2).
Lymph node metastasis was detected in 23 (48.9%) and 148 (80.4%) breast cancers in the BRCA carrier and non-BRCA carrier groups, respectively, and was more common in the non-BRCA carrier group with a statistically significant difference (p < 0.001).
When comparing the morphologic appearance and enhancement type of the BRCA1 carrier group and the BRCA2 carrier group (Tables 4 and 5), we found that there was no significant difference between the two groups regarding mass and non-mass lesions of breast cancers (Table 5). The dominant enhancement curve type for both BRCA1 carriers and BRCA2 carriers was the Type 3 enhancement curve type. Breast cancers with BRCA 2 mutation tend to show a more persistent enhancement pattern than BRCA 1 mutation, although we found no statistically significant difference. The dominant MRI characteristics of both groups included the irregular shape and indistinct margin with a heterogeneous enhancement pattern. No significant differences were found between the two groups regarding the number of mass lesions and non-mass lesions (Table 6).
Mammography has been the primary screening modality for women over 40 years of age in the general population. However, patients with gene mutated breast cancer tend to develop cancer at a younger age and the dense breast tissue in these women can lower the sensitivity of cancer detection with mammography (8, 9, 11). Given the limited efficacy of mammographic surveillance of women at a high genetic risk, the most important type of imaging is breast MRI (10).
BRCA-associated breast cancer has been shown to exhibit benign morphologic features more than sporadic breast cancers and this can cause them to be confused with benign lesions; in particular, BRCA1-associated breast cancers have a tendency to imitate fibroadenomas or even cysts (12, 13). In previous report up to 30% of invasive cancers found on MRI in a high-risk patient group had smooth margins, round or oval shape, and 24% were of the benign enhancement curve type (12, 13). The reason for the high prevalence of benign morphologic features in the BRCA carrier group can be explained by the high grade of the lesion. Although we did not evaluate the grade of each cancer it is a well-known fact that most gene mutation breast cancers show a higher grade than sporadic breast cancers. Because of the rapid tumor growth, these high-grade lesions have a tendency to show small, well-circumscribed margins and a round or oval shape (14), all of which can cause them to be confused with benign breast lesions.
In our study, the MR imaging features in BRCA mutation group were mostly typical for invasive ductal carcinoma and showed an irregular shape, indistinct margin, and heterogenous enhancement with a Type 3 enhancement curve type. However, slightly more cancers in BRCA carriers had a Type 1 persistent enhancement curve and a round shape compared with those in the non-BRCA carrier group, although this difference was not significant. We also found no significant difference in tumor size or enhancement curve type with respect to the genetic status of the breast cancer. Due to the small number of the data set and the fact that the study population was limited to a single institution, we had only a limited ability to detect significant difference between the patient groups.
Lymph-node metastasis was more common in the non-BRCA carrier group and had statistical significance (p < 0.001). Generally, the BRCA carrier shows high-grade tumor features as it is more aggressive. Because our study population was limited to young patients with a strong family history of breast cancer, the control group might also have features of high-grade breast cancer. This may explain the difference in the number of lymph node metastasis in the two groups.
Comparing the pathologic tumor size of the breast cancers in BRCA1 carrier and BRCA2 carrier Gilbert et al. (15) reported that BRCA1 carriers had a larger size than BRCA2 carriers, probably due to the more rapid growth of the tumor in BRCA1 carriers. However, no significant size difference was found in our study. Unlike the previously reported data that BRCA mutated breast cancers more frequently show non-mass-like enhancement than those of the sporadic group, there was no significant statistical difference in the number of mass and non-mass lesions of the tumor (13).
Studies of breast cancer patients in Korea have led to identification of the family history in 7.6% of the patients, and the mutations in BRCA1 and BRCA2 have been found to contribute to 5-10% of genetic breast cancers in Korean women. However, previous studies, including ours, are studies from hospital-based, limited data. Large, population-based studies are still needed to establish the frequency and characteristics of hereditary breast cancer among Korean patients (1, 16).
Another limitation of our study is that as our study population was limited to young patients and those with a strong family history of breast cancer, the non-BRCA carrier group cannot completely comprise the control group.
In summary, breast cancers with BRCA1/2 gene mutation carriers tend to have benign morphologic features on MRI, such as a round shape or Type 1 kinetic curve enhancement, that are commonly seen in benign lesions. Therefore, radiologists should be suspicious of any new breast MRI lesions in a BRCA1/2 gene mutation carrier, and appropriate biopsy should be considered, if necessary.
Figures and Tables
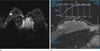
Fig. 2
a. An axial subtraction MR image of a 32-year-old BRCA 2 gene mutation carrier. In the right breast outer portion, an oval shaped, homogeneous enhancing lesion is visible with longest diameter of 8 mm. b. The kinetic analysis reveals delayed persistent enhancement of the lesion.
References
1. Choi DH, Lee MH, Bale AE, Carter D, Haffty BG. Incidence of BRCA1 and BRCA2 mutations in young Korean breast cancer patients. J Clin Oncol. 2004; 22:1638–1645.
2. Stratton MR. Pathology of familial breast cancer: differences between breast cancers in carriers of BRCA1 or BRCA2 mutations and sporadic cases. Breast cancer linkage consortium. Lancet. 1997; 349:1505–1510.
3. Feig SA, D'Orsi CJ, Hendrick RE, et al. American college of radiology guidelines for breast cancer screening. AJR Am J Roentgenol. 1998; 171:29–33.
4. Burke W, Daly M, Garber J, et al. Recommendations for follow-up care of individuals with an inherited predisposition to cancer. Ii. BRCA1 and BRCA2. Cancer genetics studies consortium. JAMA. 1997; 277:997–1003.
5. Kriege M, Brekelmans CT, Boetes C, et al. Efficacy of MRI and mammography for breast-cancer screening in women with a familial or genetic predisposition. N Engl J Med. 2004; 351:427–437.
6. Kriege M, Brekelmans CT, Boetes C, et al. MRI screening for breast cancer in women with familial or genetic predisposition: design of the Dutch National Study (MRISC). Fam Cancer. 2001; 1:163–168.
7. Plevritis SK, Kurian AW, Sigal BM, et al. Cost-effectiveness of screening BRCA1/2 mutation carriers with breast magnetic resonance imaging. JAMA. 2006; 295:2374–2384.
8. Warner E, Plewes DB, Shumak RS, et al. Comparison of breast magnetic resonance imaging, mammography, and ultrasound for surveillance of women at high risk for hereditary breast cancer. J Clin Oncol. 2001; 19:3524–3531.
9. Kuhl CK, Schmutzler RK, Leutner CC, et al. Breast MR imaging screening in 192 women proved or suspected to be carriers of a breast cancer susceptibility gene: preliminary results. Radiology. 2000; 215:267–279.
10. Saslow D, Boetes C, Burke W, et al. American cancer society guidelines for breast screening with MRI as an adjunct to mammography. CA Cancer J Clin. 2007; 57:75–89.
11. Warner E, Plewes DB, Hill KA, et al. Surveillance of BRCA1 and BRCA2 mutation carriers with magnetic resonance imaging, ultrasound, mammography, and clinical breast examination. JAMA. 2004; 292:1317–1325.
12. Veltman J, Mann R, Kok T, et al. Breast tumor characteristics of BRCA1 and BRCA2 gene mutation carriers on MRI. Eur Radiol. 2008; 18:931–938.
13. Schrading S, Kuhl CK. Mammographic, US, and MR imaging phenotypes of familial breast cancer. Radiology. 2008; 246:58–70.
14. Blaichman J, Marcus JC, Alsaadi T, El-Khoury M, Meterissian S, Mesurolle B. Sonographic appearance of invasive ductal carcinoma of the breast according to histologic grade. AJR Am J Roentgenol. 2012; 199:W402–W408.
15. Gilbert FJ, Warren RM, Kwan-Lim G, et al. Cancers in BRCA1 and BRCA2 carriers and in women at high risk for breast cancer: MR imaging and mammographic features. Radiology. 2009; 252:358–368.
16. Son BH, Ahn SH, Park SK, Kim SW. Hereditary breast cancer in Korea: a review of the literature. J Breast Cancer. 2008; 11:1–9.




 PDF
PDF ePub
ePub Citation
Citation Print
Print









 XML Download
XML Download