Abstract
Although most cases of posterior reversible encephalopathy syndrome (PRES) are reversible, irreversible lesions as a form of hemorrhage or infarction have been described. PRES as a complication of systemic lupus erythematosus (PRES-SLE) is associated with hypertension or use of immunosuppressive agents. We present a case of recurrent atypical PRES-SLE, which showed restricted diffusion in the first manifestation of SLE, resulted in parenchymal hemorrhagic transformations in the recurrent episode.
A diagnosis of posterior reversible encephalopathy syndrome (PRES) is based on a combination of clinical symptoms including headache, altered alertness, abnormalities of visual perception, and seizure, and characteristic neuroimaging findings of transient white matter edema, mostly in the posterior parietal-temporal-occipital regions of the brain (1, 2). Conditions commonly associated with PRES include severe hypertension, eclampsia/preeclampsia, immunosuppressive medications including cyclosporine and taclorimus, antineoplastic agents, and various cause of renal failure (3, 4).
PRES as a complication of systemic lupus erythematosus (PRES-SLE) is not uncommon and neurologic or psychiatric abnormalities occur in up to two-thirds of patients with SLE (4). Usually PRES is reversible vasogenic edema in the posterior circuation territories, although atypical findings such as conversion to irreversible cytotoxic edema, hemorrhage, and enhancement have been described (3). And cytotoxic edema surrounded by extensive vasogenic edema resulted in poor outcome. We report a case of PRES-SLE showing irreversible hemorrhagic transformation as a complication of recurrent PRES.
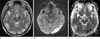
A 29-year-old woman without any specific disease history presented general tonic clonic seizure. Brain magnetic resonance imaging (MRI) demonstrated increased signal intensities in the gray and white matter of parieto-temporo-occipital lobes on T2-weighted images (T2-WI) and fluid attenuated inversion recovery (FLAIR) (Fig. 1). Most of the lesions exhibited high values on the apparent diffusion coefficient (ADC) map, however area of diffusion restriction was seen in the gray matter of left temporoocciptal lobe. MRA showed no demonstrable vascular abnormality. She was diagnosed with PRES and provided with intensive control of blood pressure with IV antihypertensive agent.
She had proteinuria, a creatinine of 1.2 mg/dL. The laboratory studies disclosed leukocytes 15 900/mm3, hemoglobulin 7.2 g/dL, platelets 179 000/mm3, serum albumin 2.5 g/dL, C-reactive protein 2.2 mg/dL (reference, < 0.5), positive antinuclear antibodies (ANA) and anti-double stranded DNA antibodies (anti-dsDNA), and a low serum complement (C3: 24.3 mg/dL, reference, 90-180; C4: 2.1 mg/dL, reference, 10-40). Anti-cardiolipin, anti-phospholipid and lupus anticoagulant antibodies were negative and coagulation tests were normal. And she diagnosed lupus nephritis (WHO class IV) by renal biopsy. The patient did not experience chills or fever, and no evidence of viral or bacterial infection after extensive evaluation, including blood cultures, HIV and syphilis serology.
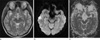
She was treated with methylprednisolone and cyclophosphamide pulse therapy followed by high doses of cyclosporine. Her systolic blood pressure was normalized to less than 130 mmHg, and her neurological symptoms resolved completely. Pancytopenia, developed after cyclophosphamide pulse therapy, was corrected by neutrogin. The bilateral posterior cerebral lesions had disappeared on follow-up MRI 1 month later (Fig. 2).
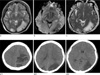
2 months later, she suddenly developed hypertension (190/130 mmHg), headache, a generalized seizure, and then confusion, vertigo and slurred speech. MRI showed extensive edematous lesions in the left frontoparietal and temporooccipital lobes and right occipital lobe on T2WI and FLAIR. And dark signal intensity spots suggestive hemorrhagic transformation are contained within the left occipital lesion, previously shown cytotoxic edema, and left parietal lobe (Fig. 3). Most of the lesions except small gliotic change were resolved on follow-up MRI.
DWI may be helpful for distinguishing arterial ischemic injury (characterized by a reduced ADC) from vasogenic brain edema (characterized by increased ADC), which is characteristically seen in PRES (5). However, a few case reports have described cytotoxic edema as a complication of PRES (6). Covarrubias et al. (6) reported that six of 22 PRES patients showed areas of high DWI signal intensity with normal ADC values, which they called pseudonormalized, surrounded by abnormal T2 signal intensity and high ADC values. The high DWI signal intensity and pseudonormalization were associated with an adverse outcome, and so might represent the earliest sign of non reversibility as severe vasogenic edema progressees to cytotoxic edema.
The pathophysiology of PRES is not clear. Severe hypertension with failed auroregulation, leading to hyperperfusion and breakthrough brain edema remains more popular as a potential cause of the vasogenic edema. Alternatively, cerebral autoregulatory vasoconstriction, ischemica, hypoperfusion, and subsequent brain edema have been demonstrated and could account for the watershed pattern (3, 7, 8). Hinchey et al. (1) identified 2 patients who developed PRES-SLE and suggested that the mechanism of PRES was a brain-capillary leak syndrome related to hypertension, fluid retention, and the cytotoxic effects of immunosuppressive agents on the vascular endothelium. In our case, the patient was hospitalized with generalized seizure and showed hypertension and oliguria. She had no medication history for immunosuppressive agent. Therefore the 1st episode of PRES-SLE seemed to be associated with hypertension and fluid retention. However, area of diffusion high signal intensity with decreased ADC was seen in the gray matter of the left temporooccipital lobe, which showed near complete resolution on the follow-up MRI. MRA was normal. The pathophysiology of the PRES-SLE with diffusion restriction is not clear, but both of the two hypotheses may be available.
Recurrent episode of PRES is not common, and the incidence is unknown. To our best knowledge, there was only one case report for recurrent PRESS-SLE (9). In the case report, MRA showed narrowing segment of right M1 and left A2, representing either vasospasm or vasculitis. DWI showed normal. Sweany et al. (8) reported 3 recurrent cases (3.8%) of 78 PRES patients in a retrospective review, and was associated with sickle-cell disease with infection, antibody-positive autoimmune disease and allogeneic bone marrow transplantation with infection. Through the comparison of the clinical features in the 3 patients, they emphasized the importance of infection, multiple organ dysfunction syndrome, and endothelial injury in PRES. Although most occurrences of PRES are reversible, irreversible lesions can occur as a form of hemorrhage or infarction. In most cases of PRES, clinical symptoms and neuro images resolved with aggressive blood pressure management and removal of the causative factors. Immediate recognition and correction of a condition that provokes PRES is the best way to treat the disorder (1, 2).
Prior studies have demonstrated parenchymal hematoma or sulcal subarachnoid hemorrhage in 5-17% of patients with PRES (3, 10), but the mechanism behind hemorrhage in PRES is equally unclear (3). In our case, petechial hemorrhagic lesions with extensive vasogenic edema were developed in the left occipital lobe, previously shown cytotoxic edema, and left parietal lobe at the recurrent attack. She was on steroid and cyclosporin treatment for the lupus nephritis and showed post-chemotherapy pancytopenia. Cyclosporin-induced immunosuppression and endothelial injury and pancytopenia induced by chemotherapy might be the cause of the recurrence and hemorrhagic lesions.
We present the first case of hemorrhagic transformation as a recurrent PRES in a patient with SLE who presented atypical radiologic features of PRES including diffusion restriction. In the management of PRES, medication of cyclosporin should be considered carefully especially in the case of having diffusion restriction within extensive vasogenic edema.
Figures and Tables
Fig. 1
A 29-year-old woman with SLE. T2-weighted image of magnetic resonance imaging shows increased signal intensities in the gray and white matter of parieto-temporo-occipital lobes (a). Most of the lesions exhibited high values on the apparent diffusion coefficient map, however area of diffusion restriction was seen in the gray matter of left temporoocciptal lobe on diffusion weighted image (b) and apparent diffusion coefficient map (c).
References
1. Hinchey J, Chaves C, Appignani B, et al. A reversible posterior leukoencephalopathy syndrome. N Engl J Med. 1996; 334:494–500.
2. Kinoshita T, Moritani T, Shrier DA, et al. Diffusion-weighted MR imaging of posterior reversible leukoencephalopathy syndrome: a pictorial essay. Clin Imaging. 2003; 27:307–315.
3. McKinney AM, Short J, Truwit CL, et al. Posterior reversible encephalopathy syndrome: incidence of atypical regions of involvement and imaging findings. AJR Am J Roentgenol. 2007; 189:904–912.
4. Magnano MD, Bush TM, Herrera I, Altman RD. Reversible posterior leukoencephalopathy in patients with systemic lupus erythematosus. Semin Arthritis Rheum. 2006; 35:396–402.
5. Provenzale JM, Petrella JR, Cruz LC, Wong JC, Engelter S, Barboriak DP. Quantitative assessment of diffusion abnormalities in posterior reversible encephalopathy syndrome. AJNR Am J Neuroradiol. 2001; 22:1455–1461.
6. Covarrubias DJ, Luetmer PH, Campeau NG. Posterior reversible encephalopathy syndrome: prognostic utility of quantitative diffusion-weighted MR images. AJNR Am J Neuroradiol. 2002; 23:1038–1048.
7. Bartynski WS. Posterior reversible encephalopathy syndrome, part 2: controversies surrounding pathophysiology of vasogenic edema. AJNR Am J Neuroradiol. 2008; 29:1043–1049.
8. Sweany JM, Bartynski WS, Boardman JF. "Recurrent" posterior reversible encephalopathy syndrome: report of 3 cases--pres can strike twice. J Comput Assist Tomogr. 2007; 31:148–156.
9. Thaipisuttikul I, Phanthumchinda K. Recurrent reversible posterior leukoencephalopathy in a patient with systemic lupus erythematosus. J Neurol. 2005; 252:230–231.
10. Lee VH, Wijdicks EF, Manno EM, Rabinstein AA. Clinical spectrum of reversible posterior leukoencephalopathy syndrome. Arch Neurol. 2008; 65:205–210.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download