Abstract
It is a well-known clinical fact that contrast-enhanced magnetic resonance angiography exaggerates vertebral arterial ostial stenosis and sometimes shows pseudostenosis. Considering the clinical significance of a lesion in the posterior circulation ischemia, the importance of an accurate imaging diagnosis of ostial stenosis should not be underestimated. We were able to differentiate pseudostenosis of the ostium from true stenosis using thin-slab maximum-intensity-projection(MIP) images which are thought to be helpful for minimizing standard full thickness MIP images.
Contrast-enhanced magnetic resonance angiography (CE-MRA) is a highly sensitive technique used to detect cervical artery stenosis; it has become part of the routine imaging for detecting brain stroke as well as atherosclerotic diseases (1-4). This technique produces arterial images with high-spatial resolution and which are free from the flow-related artifacts of time-of-flight (TOF) MRA. However, owing to the technical limitations of the currently available CE-MRA technique, there are numerous disadvantages including an image acquisition timing issue, its relatively low spatial resolution, overestimation of the degree of stenosis, etc. It has been suggested that the relatively low spatial resolution and inherent limitations of the maximum-intensity-projection (MIP) technique are responsible for the overestimation of a certain stenosis in CE-MRA. Pseudostenosis of the vertebral artery (VA) ostium is also one of the common problems encountered when using this technique which may exaggerate preexisting focal stenosis of the ostium or show a false stenosis of an otherwise intact vessel (3, 5). It has been reported that lesion overestimation is seen in 15% of cases (3). Although the mechanism of the pseudostenosis is not well understood, it is said that turbulent flow in the particular anatomic region causes intravoxel signal dephasing which results in decreased signal intensity. Because of the difference in the signal intensity of the ostial region from that of the other segments of the VA, this particular region appear stenotic on 3-dimensional (3D), full-thickness MIP images (MIP artifact). Recent improvement of the image quality has decreased this problem, however, it is still a frequently seen phenomenon with the current imaging techniques.
As we found that thin-slab (slab thickness of 1~2 mm) MIP was very helpful for minimizing the MIP artifact, we began to apply this simple method for every lesion noted on routine MIP images. The purpose of this short technical report is to present our method of applying the thin-slab MIP technique, as it is very helpful for minimizing pseudostenosis of the VA os.
MR imaging was performed on a 3.0-T MR system (Gyroscan Intera Achieva, Philips Medical System, The Netherlands) equipped with a neurovascular head and neck coil. High-spatial-resolution 3D CE-MRA was obtained in the coronal plane using a fast-field, gradient-echo sequence together with the contrast-enhanced timing robust angiography (CENTRA) k-space acquisition technique which is a modified centric k-space ordering technique. The sequence was performed with 200 coronal partitions, each with a 0.45-mm slice thickness, 5/2 msec [TR/TE], 3 NEX, 3° flip angle, 320-mm3 field of view according to a 512 × 512 image matrix, factor 3 sensitivity encoding (SENSE), and an imaging time of 69 seconds. Reconstruction was performed without zero filling. The spatial resolution was 0.63 × 0.63 × 0.90 mm3. Breath-holding was not used. The contrast material (Dotarem [0.5 mmol/mL], Laboratoire Guerbet, Aulnay-su-Bois, France) was infused by a 22-gauge venous angiocatheter via the antecubital fossa by using a Medrad Spectris Solaris® MR Injection System (USA). 0.2 milliliters/kg of contrast material was injected at a rate of 2 mL/sec. Each bolus was immediately followed by a 20-mL saline flush.
For the routine MRA image display, the images were post-processed on a ViewForum workstation (Philips Medical System, The Netherlands). Post-processing sub-volumes were generated using the MR post-processing console to create 15, maximum-intensity-projection (MIP) images at 12° increments each on both the horizontal and vertical axes. MRA source images were temporarily stored in a mini-PACS system (TeraRecon, San Mateo, CA).
When any arterial lesion was seen on the routine projections, a radiologist generated reformatted images using the source images archived on the mini-PACS system. We used a PC-based, 3D image processing software (Aquarius NET 1.8.2.6 ; TeraRecon, San Mateo, CA). When there was any stenosis at the VA os, we re-evaluated the degree of stenosis using thin-slab MIP images (slab thickness of 1~2 mm) with variable direction and which followed the course-of VA from the os to the proximal VA. A radiologist performed a qualitative analysis comparing the images of the VA ostial stenosis with MR angiogram and digital subtraction angiography (DSA). With this interactive review of the stenosis, we could ascertain whether the stenosis was true or false as can be seen in Figures 1 and 2.
In the posterior circulation, evaluation of clinically relevant stenosis remains difficult with MR angiography, primarily because of the small diameter of the vertebral arteries and the frequent anatomic variations with variable direction. Randoux et al. reported that MR angiography was a useful method to rule out ostial stenosis of the craniocervical vessels except vertebral artery. They observed that 15% of VA os stenosis was misclassified due to overestimation of the stenosis and that the positive predictive values of MRA for VA os stenosis greater than 50% was 58% (3).
We thought that the possible reason for the MIP artifact in that specific region was regional low signal intensity of the VA os which was probably caused by the intravoxel dephasing phenomenon (4, 6). The cause of intravoxel dephasing is not yet well understood. This phenomenon is due to the spin dephasing when the voxel size is comparable to the residual lumen at level of stenosis (7). In addition to, previous studies were explained that turbulent flow in the axilla area of a small vessel may cause an intravoxel dephasing, especially at a broad bifurcation angle (8, 9). To reduce the intravoxel dephasing effect, we have to obtain the CE-MRA using a smaller voxel size, but does not yield in a stenosis grading improvement (7). Also, thick slab MIP image can lead to overestimate stenoses because of partial volume averaging effect (10).
Because of the frequent exaggeration of stenosis in this area, we tend to underestimate the apparent stenosis of VA os in our daily clinical practice. However, considering the high incidence of the VA origin of atherosclerotic disease, as was observed in the New England Medical Center Posterior Circulation Registry (NEMC-PCR) (131 of 407, 32%) and the frequent association of posterior circulation infarction and significant stenosis of the VA os, we must give more attention to this lesion (11). For these reasons, VA os stenosis was overestimated on MIP images of CE-MRA. Therefore, we tend to underestimate the degree of VA os stenosis in our daily clinical practice. However, VA os stenosis (> 50%) is very common and is attributable to artery-to-artery emboli in the NEMC-PCR If we consider the tendency toward exaggeration on CE-MRA, we may neglect a significant source of incorrectly diagnosed VA os stenosis. However, performing digital subtraction angiography is not a good clinical option simply for confirmation of possible stenosis as this procedure can have distinct negative ramifications (12).
Considering these problems, review of source images also required additional access to the special PACS image data serve system. But, a simple, PC-based review of a lesion using a thin-slab MIP technique is very helpful in order to both minimize possible exaggeration of the stenosis on images as well as the potential clinical underestimation of a possible, existing stenosis. CNR of vessels on MIP images decrease with increasing background signal intensity and reduce vessel size. Thin-slap MIP image can be effect, as it leads to minimize inclusion of background tissue into the slab (10). So, thin-slab MIP image will be beneficial to stenosis grading, so as to include as few voxels as possible in the slab. Also, it is possible to represent a vessel with different orientation.
However, differentiation of VA ostial stenosis was not always possible because of the technical limitations of the technique in some patients. It is very difficult to determine the degree of stenosis if the signal-to-noise ratio is too low and this is not uncommon in those regions. Tracing the course of a very tortuous, proximal VA is sometimes quite difficult.
Figures and Tables
Fig. 1
(a) Oblique digital subtraction angiography image. (b) Oblique gadolinium-enhanced sagittal thick (40-mm) MR angiography. (c) Thin-slab MIP (slab thickness of 1 mm) image. Digital subtraction andgiography and MR angiography show concordant results of severe ostial stenosis (arrow) of the left VA. The thin-slab MIP image shows no signal in the left VA os. This case represents true VA ostial stenosis.
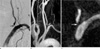
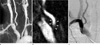
Fig. 2
(a) The findings on oblique gadolinium-enhanced MR angiography shows a loss of signal in the right VA os, which is considered to represent severe stenosis. (b) Thin-slab MIP (slab thickness of 1 mm) image shows a mild signal defect (arrowhead) in the right VA os and intact luminal patency of the right VA os. (c) Oblique DSA image shows no significant VA stenosis. Using the thin-slab MIP image, we were able to differentiate the pseudostenosis from true stenosis of the right VA os.
References
1. Kollias SS, Binkert CA, Ruesch S, Valavanis A. Contrast-enhanced MR angiography of the supra-aortic vessels in 24 seconds: a feasibility study. Neuroradiology. 1999. 41:391–400.
2. Phan T, Huston J 3rd, Bernstein MA, Riederer SJ, Brown RD Jr. Contrast-enhanced magnetic resonance angiography of the cervical vessels: experience with 422 patients. Stroke. 2001. 32:2282–2286.
3. Randoux B, Marro B, Koskas F, Chiras J, Dormont D, Marsault C. Proximal great vessels of aortic arch: comparison of three-dimensional gadolinium-enhanced MR angiography and digital subtraction angiography. Radiology. 2003. 229:697–702.
4. Remonda L, Senn P, Barth A, Arnold M, Lovblad KO, Schroth G. Contrast-enhanced 3D MR angiography of the carotid artery: comparison with conventional digital subtraction angiography. AJNR Am J Neuroradiol. 2002. 23:213–219.
5. Leclerc X, Martinat P, Godefroy O, et al. Contrast-enhanced three-dimensional fast imaging with steady-state precession (FISP) MR angiography of supraaortic vessels: preliminary results. AJNR Am J Neuroradiol. 1998. 19:1405–1413.
6. Slosman F, Stolpen AH, Lexa FJ, et al. Extracranial atherosclerotic carotid artery disease: evaluation of non-breath-hold three-dimensional gadolinium-enhanced MR angiography. AJR Am J Roentgenol. 1998. 170:489–495.
7. Cosottini M, Calabrese R, Puglioli M, et al. Contrast-enhanced three-dimensional MR angiography of neck vessels: does dephasing effect alter diagnostic accuracy? Eur Radiol. 2003. 13:571–581.
8. Chung TS, Lee YJ, Kang WS, et al. Clinical and experimental investigation of pseudoaneurysm in the anterior communicating artery area on 3-dimensional time-of-flight cerebral magnetic resonance angiography. J Comput Assist Tomogr. 2004. 28:414–421.
9. Evans AJ, Richardson DB, Tien R, et al. Poststenotic signal loss in MR angiography: effects of echo time, flow compensation, and fractional echo. AJNR Am J Neuroradiol. 1993. 14:721–729.
10. Neri E, Cosottini M, Caramella D. MR angiography of the body: technique and applications. 2010. Heidelberg: Springer;36–39.
11. Caplan LR, Wityk RJ, Glass TA, et al. New England Medical Center Posterior Circulation registry. Ann Neurol. 2004. 56:389–398.
12. Hankey GJ, Warlow CP, Sellar RJ. Cerebral angiographic risk in mild cerebrovascular disease. Stroke. 1990. 21:209–222.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download