Abstract
Purpose
To describe the imaging findings at susceptibility weighted imaging (SWI) at 3T in patients with occlusion of middle cerebral artery, and to correlate the absence or presence of arterial bright foci in sylvian fissure, as one of their finding at SWI, with the diffusion weighted imaging (DWI) scores.
Materials and Methods
We included 12 patients with symptomatic unilateral occlusion of middle cerebral artery. Retrospective review of SWI and DWI was done. On DWI, size of infarction was analyzed according to the ASPECTS grading system. On SWI, presence of hemorrhage, dark blooming of intravascular clot, distension of medullary or cortical vein, and absence or presence of bright arterial foci in sylvian fissure were evaluated.
Results
Of 12 patients with symptomatic unilateral MCA occlusion, SWI showed dark blooming of intravascular clot in 8 patients (66.7%), distended medullary or cortical vein in 7 patients (58.3%), nonvisualization of arterial bright signal intensity in sylvian fissure in 7 patients (58.3%), and hemorrhage in one patient (8.3%). In comparison with DWI, patients with sylvian arterial bright signal intensity showed better ASPECTS score (6.4±4.1) than patients without arterial bright signal intensity (4.4±1.1), yet it was not statistically significant (p=0.267, t-test).
Stroke can be defined as an acute central nervous system injury with an abrupt onset. Acute ischemia constitutes most part of stroke and is a major cause of mortality and morbidity. Large vessel occlusions, such as internal carotid artery (ICA), middle cerebral artery (MCA) and posterior cerebral artery (PCA), constitute approximately 50% of all acute cerebral infarction. Among the rest, MCA is a leading portion of cerebral vessel occlusion (1, 2).
Early diagnosis is the goals of an imaging evaluation for acute stroke. Another goal of an imaging evaluation is to obtain accurate information about the intracranial vasculature and brain perfusion for guidance in selecting the appropriate therapy. A full evaluation may be done with a combination of computed tomography (CT) or magnetic resonance (MR) imaging techniques. The introduction of new MRI techniques has touched up acute stroke diagnosis. Diffusion- and perfusion-weighted sequences and magnetic resonance angiography (MRA) provide data on the pathophysiology of ischemia and may contribute to therapeutic decisions.
Susceptibility-weighted imaging (SWI) also adds informations about acute stroke. Susceptibility weighted imaging (SWI) is a magnetic resonance (MR) technique, which is sensitive to paramagnetic substances, such as deoxygenated blood, blood products, iron, and calcium (3). This technique has been described in variety of neurological disorders, such as trauma, tumors, vascular malformations, multiple sclerosis, venous thrombosis, and stroke (4). SWI can be a useful MR sequence in the work-up of stroke patients, the characteristic imaging findings often may be seen only in this sequence, such as detection of intracranial hemorrhage, hemorrhagic transformation of acute stroke and detection of intraarterial thrombus. SWI at higher field strength has benefits of increased sensitivity to altered susceptibility (4), but there is limited number of reports regarding the description of SWI findings in patients with acute stroke. The purpose of this study is to describe the SWI findings in patients with the occlusion of middle cerebral artery, and to correlate them with diffusion weighted imaging findings.
The study was institutional review board approved, and a waiver of consent was obtained for a Health Insurance Portability and Accountability Act-compliant retrospective study. Twelve patients (3 men, 9 women; age range 44-90; mean age 71) who experienced MCA territory stroke symptoms and had occlusion of the unilateral MCA on MR angiography (MRA) and infarction in the MCA territory on DWI were included in this study. Nonvisualization of entire M1 segment of the MCA on MRA was considered as arterial occlusion, and patients with partial segment loss of arterial flow signal was excluded due to the possibility of severe stenosis without occlusion. The mean duration between symptom onset and acquisition of SWI and DWI was 7.9 hours (range 0.5-36).
SWI and DWI were obtained with 3T MR unit (Verio, Siemens Medical Solutions, Erlangen, Germany). SWI acquisition was performed with a T2* weighted gradient echo sequence with FA=15°, TR/TE=28/20 ms, matrix number=256×182, FOV=21 cm×21 cm, slice thickness=2 mm. SWI and minimum intensity projection (mIP) images were acquired by in-line postprocessing of magnitude and phase images, and these post-processed mages were used for the evaluation of the imaging findings in this study. DWI was performed with single-shot echo-planar imaging (ss-EPI), and parameters were as follows; b-value 1000 s/mm2, TR/TE 5800/99 ms, slice thickness 4 mm, FOV 23 cm×23 cm, matrix 128×128, NEX 1.
Two experienced neuroradiologists (each with over 10 years experience) interpreted the SWI and DWI. Four imaging findings were evaluated on SWI ; 1) arterial blooming at the thrombosed segment of occluded MCA, 2) presence or absence of bright arterial signal intensity in sylvian fissure, 3) prominence of medullary or cortical vein at the MCA territory compared to the opposite side, and 4) presence of parenchymal hemorrhage were studied. To evaluate infarction size on DWI, the areas of bright signal intensity on DWIs were scored according to the criteria of Alberta Stroke Program Early CT Score (ASPECTS) (5).
12 consecutive patients were identified from the radiology data base who were diagnosed with MCA occlusion on CTA, MRA or DSA and underwent SWI on a 3T magnet from March to August 2009 (Table 1).
Table 2 summarizes the MR findings for SWI and DWI in the 12 patients with symptomatic unilateral MCA occlusion.
Signal loss at the occluded vessel was seen in 8 patients (66.7%), and it exceeded true MCA diameter (blooming). At the territory of occluded MCA, distended medullary vein or cortical vein was seen in 7 patients (58.3%). There was one patient (8.3%) with early hemorrhagic transformation seen as inhomogeneous dark signal intensity with mass effect.
Arterial bright signal intensity in the sylvian fissure distal to the occluded M1 segment of MCA was seen in 5 patients (41.7%). In comparison of patients with or without arterial bright signal intensity, ASPECTS score was higher in patients with arterial bright signal intensity in the sylvian fissure (mean ± SD = 6.4 ± 4.1) than those without arterial bright signal intensity (mean ± SD = 4.4 ± 1.1), but it was not statistically significant(p=0.267, t-test). Two patients with infarction involving entire MCA territory (ASPECTS=0) showed total loss of arterial bright signal intensity in sylvian fissure.
SWI is a fully velocity-compensated, three-dimensional (3D), gradient-echo (GRE) sequence that uses both phase and magnitude data to achieve intense sensitivity to tissue magnetic susceptibility effects. The phase and magnitude data are acquired. The substances which have different magnetic susceptibilities compared to adjacent tissues result in the phase differences, that is the basis of the contrast between brain versus blood, iron laden tissues and venous structures (3, 6, 7). The phase images are used to create a phase mask after unwrapping and high pass filtering, that is then multiplied with the magnitude images to enhance the conspicuity of paramagnetic substance (8).
Most frequent SWI finding in patients with symptomatic unilateral MCA occlusion, in our study, was arterial blooming of clot in occluded vessel (Fig. 1). The term of "blooming" is used for emphasis or to express annoyance. Susceptibility-based perfusion MRI demonstrates thrombosed middle cerebral artery as signal loss along the course of artery. It is called also as MCA magnetic susceptibility sign (9). This susceptibility change is ascribed to the high deoxyhemoglobin content of fresh clots. This sign may be correlated with the hyperdense MCA sign at nonenhancing CT. Chalela et al also briefly reported it on the hypointense MCA sign at gradient-echo (GRE) imaging (10). The susceptibility effect is also named as arterial "blooming" because the effect exceeds the true MCA diameter (11) (Fig. 1).
The outcome of tissue at risk for necrosis in stroke patient depends on the presence of an efficient collateral blood flow (12). Previously, the assessment of leptomeningealcollateral circulation in acute stroke mainly relies on the analysis of perfusion or contrast enhanced image for identification of collaterally perfused areas (13). On susceptibility-weighted MR image at 3T in our series, the leptomeningeal collateral vessels are demonstrated as bright dots in sylvian fissure (Fig. 2). This arterial bright signal intensity is thought to be of time-of-flight effect from 3D gradient echo sequence. In our series, two patients with infarction involving entire MCA territory (ASPECTS=0) showed total loss of this arterial bright signal intensity. Furthermore, ASPECTS score was higher in patients with arterial bright signal intensity in sylvian fissure (range 5-9, median 7) than those without arterial signal intensity (range 0-8, median 3) in our series, although it was not statistically significant. This finding can represent the visualization of efficient leptomeningeal collaterals in sylvian fissure because patients exhibiting this sign had smaller infarct volumes at baseline. Because number of patients in our study was too small to show statistical significance, further study for demonstration of correlation between collateral flow and spared arterial bright signal intensity distal to the occluded MCA on SWI should be warranted.
Deoxygenated hemoglobin is paramagnetic in SWI with a hypointense signal. Decreased arterial blood flow will also cause an increase in the amount of deoxyhemoglobin (11, 14). The oxygen extraction fraction (OEF) is defined as the ratio of deoxyhemoglobin to oxyhemoglobin in the capillaries and venous compartments. In stroke patient, the oxygen extraction fraction is markedly increased in the penumbra following arterial occlusion. Therefore this high OEF of the cortical veins increase the conspicuousness of draining cortical veins in the area of decreased perfusion. On susceptibility-weighted imaging, prominent asymmetrical cortical veins (Fig. 3) may represent possibility of being decreased perfusion including penumbra.
While the gold standard for detecting hemorrhage in stroke patients is considered as computed tomography (CT), the magnetic resonance imaging (MRI) is coming out as a reliable tool for the detection of bleeding. Susceptibility-weighted imaging (SWI) is extremely sensitive in detecting hemorrhage (4). Thus SWI helps to differentiate between ischemic and hemorrhagic strokes. This sequence also can contribute decision-making in revascularization therapies and assessment of cerebral hemodynamics following stroke (15).
In our cases, only one among the 12 consecutive patients shows hemorrhage associated with acute cerebral infarction although intracerebral hemorrhage/microbleeding or hemorrhagic transformation is not rare condition during acute stroke. Hemorrhagic transformation of stroke is observed in approximately 20~40% of all stroke patients within the first week of onset (16), and could be a devastating complication if the patient is considered for revascularization therapies. Conventional MRI often fails to detect microbleeding or small amount of intracerebral hemorrhage, but susceptibility weight MR sequences is more sensitive to detect the hemorrhages in the early state (10). The deoxyhemoglobin, converting from extravasated hemoglobin, is a paramagnetic substance and causes local magnetic field inhomogeneity. It is more detectable on susceptibility-weighted sequences, because SWI is exquisitely sensitive to magnetic field inhomogeneity. So small bleeds within the infarcted area can be detected on SWI MR sequence (17).
Our study contains several limitations. First, retrospective observation was done for relatively small number of case. Second, patients with acute MCA occlusion beyond 4 hours from onset are included in this study. Further study with larger number of the patients evaluated within 4 hours from onset of symptom would be needed for proper determination of clinical implication of SWI in the management of the hyperactue stroke patient.
In conclusion, susceptibility-weighted MR imaging at 3T is useful in the evaluation of acute stroke patients. In current clinical practice, this sequence provides relevant information about MCA occlusion as well as other vessel-originate acute cerebral infarction. Susceptibility-weighted technique also assists to diagnosis in various stroke-related conditions.
Figures and Tables
Fig. 1
Arterial blooming. 3-dimensional time-of-flight MRA (a) shows occlusion of right middle cerebral arteries. Dark signal intensity of clot in right middle cerebral artery is demonstrated on susceptibility-weighted MR image (b). The sus-ceptibility effect, attributed to clot deoxyhemoglobin content, exceeds the true MCA diameter.
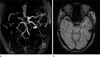
Fig. 2
Loss of arterial bright signal intensity in sylvian fissure. Diffusioin weight MR imaging (a) shows restricted diffusion area, representing acute infarction, involving right insular region. In the slice of more upper portion (b), restricted diffusion areas are also noted in the cortical and subcortical area. On magnified image of SWI (c), loss of tiny arterial bright signal foci are demonstrated in the right sylvian fissure as compared with the left side.
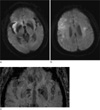
Fig. 3
Prominent veins. Diffusion weighted image (a & b) shows an acute infarct in the cortical and subcortical layer of left insula andfrontoparietal lobe. On SWI (c & d), cortical veins are prominent over the corresponding area.
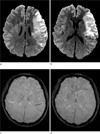
Table 2
SWI Findings and NIHSS in Patients with MCA Occlusion

Note.- Alberta Stroke Program Early CT Score (ASPECTS) was devised to quantify the extent of early ischaemic changes in the middle cerebral artery territory on CT images by using a 10-point topographic scoring system. According to this system, the MCA territory is divided into 10 regions, each of which accounts for one point in the total score. One point is deducted from that score when each area involved in stroke.
References
1. Wong KS, Ng PW, Tang A, et al. Prevalence of asymptomatic intracranial atherosclerosis in high-risk patients. Neurology. 2007. 68:2035–2038.
2. Adams HP Jr, Bendixen BH, Kappelle LJ, et al. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in Acute Stroke Treatment. Stroke. 1993. 24:35–41.
3. Haacke EM, Xu Y, Cheng YC, et al. Susceptibility weighted imaging (SWI). Magn Reson Med. 2004. 52:612–618.
4. Thomas B, Somasundaram S, Thamburaj K, et al. Clinical applications of susceptibility weighted MR imaging of the brain - a pictorial review. Neuroradiology. 2008. 50:105–116.
5. Barber PA, Demchuk AM, Zhang J, et al. Validity and reliability of a quantitative computed tomography score in predicting outcome of hyperacute stroke before thrombolytic therapy. ASPECTS Study Group. Alberta Stroke Programme Early CT Score. Lancet. 2000. 355:1670–1674.
6. Rauscher A, Sedlacik J, Barth M, et al. Magnetic susceptibility-weighted MR phase imaging of the human brain. AJNR Am J Neuroradiol. 2005. 26:736–742.
7. Xu Y, Haacke EM. The role of voxel aspect ratio in determining apparent vascular phase behavior in susceptibility weighted imaging. Magn Reson Imaging. 2006. 24:155–160.
8. Sehgal V, Delproposto Z, Haacke EM, et al. Clinical applications of neuroimaging with susceptibility-weighted imaging. J Magn Reson Imaging. 2005. 22:439–450.
9. Flacke S, Urbach H, Keller E, et al. Middle cerebral artery (MCA) susceptibility sign at susceptibility-based perfusion MR imaging: clinical importance and comparison with hyperdense MCA sign at CT. Radiology. 2000. 215:476–482.
10. Chalela JA, Haymore JB, Ezzeddine MA, et al. The hypointense MCA sign. Neurology. 2002. 58:1470.
11. Hermier M, Nighoghossian N. Contribution of susceptibility-weighted imaging to acute stroke assessment. Stroke. 2004. 35:1989–1994.
12. Bozzao L, Fantozzi LM, Bastianello S, et al. Early collateral blood supply and late parenchymal brain damage in patients with middle cerebral artery occlusion. Stroke. 1989. 20:735–740.
13. Jung SL, Lee YJ, Ahn KJ, et al. Assessment of collateral flow with multi-phasic CT: correlation with diffusion weighted MRI in MCA occlusion. J Neuroimaging. 2011. 21:225–228.
14. Yen JC, Lai YJ, Chan L. Middle cerebral artery susceptibility sign and venous prominence in acute ischemic stroke. Neurol India. 2010. 58:620–621.
15. Santhosh K, Kesavadas C, Thomas B, et al. Susceptibility weighted imaging: a new tool in magnetic resonance imaging of stroke. Clin Radiol. 2009. 64:74–83.
16. Moulin T, Crepin-Leblond T, Chopard JL, et al. Hemorrhagic infarcts. Eur Neurol. 1994. 34:64–77.
17. Nandigam RN, Viswanathan A, Delgado P, et al. MR imaging detection of cerebral microbleeds: effect of susceptibility-weighted imaging, section thickness, and field strength. AJNR Am J Neuroradiol. 2009. 30:338–343.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download