Abstract
Cardiac myxoma is the most common benign tumor of the heart. However, low incidence of recurrence and metastasis has been reported. A 49-year-old female patient was admitted in the hospital due to sudden onset of left side weakness. Magnetic resonance imaging (MRI) of brain showed multifocal areas of diffusion restriction on diffusion weighted images. Echocardiography was performed to evaluate the cause of embolic brain infarction and cardiac myxoma was found in the left atrium. The patient underwent complete excision of the mass. One year later, the patient was readmitted with symptoms of dysarthria. Brain MRI showed newly developed multiple hemorrhagic metastatic lesions. The patient underwent radiotherapy of the metastatic lesions. Although rare, cardiac myxoma can cause delayed metastasis. We report a rare case of delayed multiple cerebral metastases from the completely resected cardiac myxoma.
Cardiac myxoma is the most common benign tumor of the heart. Complete surgical removal of the myxoma is usually curative. However, low incidence of recurrence and metastasis has been reported with the brain being the most common site of cardiac myxoma metastasis (1). We report a rare case of delayed multiple cerebral metastases from the completely resected cardiac myxoma without evidence of local tumor recurrence.
A 49-year-old female patient was admitted in the hospital due to sudden onset of left side weakness. She did not have any history of medical disease or recent drug therapy. Brain magnetic resonance imaging (MRI) was performed and revealed multifocal areas of high signal intensity (SI) in bilateral cerebral hemispheres, cerebellar hemispheres, pons and right thalamus on T2-weighted images and diffusion-weighted image (DWI) which did not show enhancement on post contrast T1-weighted images. These lesions did not correlate with vascular territories, therefore, the likelihood of acute embolic infarction from cardiac origin was suggested. Cardiac echocardiography was performed to further evaluate the cause of multifocal brain infarction. On echocardiography, a large round-shaped homogenous highly mobile mass was attached to the left atrium and excision of the mass was performed under cardiopulmonary bypass. Grossly, a loose gelatinous friable mass with a broad base on atrial septum was seen and microscopic findings showed spindled and stellite cells with eosinophilic cytoplasm without significant cytologic atypia (Fig. 1). The final histopathologic diagnosis was reported as cardiac myxoma. About one year later, the patient was readmitted to the hospital with symptoms of dysarthria. Brain MRI showed newly developed multiple hemorrhagic and enhancing lesions with perilesional edema and adjacent superficial siderosis in bilateral cerebral hemispheres (Fig. 2). The previously noted multifocal embolic areas showed complete encephalomalacia in this study. Evaluation of echocardiography did not show evidence of recurrence and full evaluation was performed to rule out the possibility of brain metastases from another origin. However, no other focus of primary malignancy was found. Although histopathologic confirmation of the cerebral lesions was not performed, hemorrhagic cerebral metastases from the previously resected cardiac myxoma were suggested. The patient underwent whole brain radiation treatment 35 Gy/24 fractions for 3 weeks and follow up MRI revealed slight improvement of the multiple enhancing cerebral lesions (Fig. 3).
Cardiac myxoma is the most common benign heart tumor, most commonly found in the left atrium (1). Cardiac myxoma is usually a sporadic lesion most commonly found in women over 30 years. However, it has been also related to the autosomal dominant syndrome called Carney complex characterized by spotty pigmentation (blue nevi and lentigines), myxomas (cardiac, cutaneous, and mammary), endocrine over-activity (Cushing's syndrome and acromegaly), testicular tumors and schwannomas (2). Patients with cardiac myxoma usually present with one of the classic triad; obstructive cardiac signs, embolic signs or systemic constitutional manifestations at the time of diagnosis (3). Because most cardiac myxomas are located in the left atrium, systemic emboli are frequent and occur in 10-45% of myxoma, with more than two-thirds of myxomatous emboli migrating to the central nervous system (4). In spite of the benign nature of cardiac myxoma, it infrequently recurs at the site of the original tumor or metastasizes to extracardiac sites with the brain known to be the most frequent sites for metastasis (5). Contrary to primary myxoma, more frequently found in women, cardiac tumor recurrence seems to occur more commonly in men. The frequency of recurrences in cardiac myxomas is about 3% for sporadic cases and up to 22% for cases associated with Carney complex. Recurrence is reported to be associated with incomplete excision, multifocality and embolism of tumor fragments (6). Metastasis of cardiac myxoma is rare and is known to be intravascular and presents with delayed occurrence after resection of the cardiac lesion (1). Metastatic lesions sometimes present earlier than the diagnosis of the primary lesion, however, has been diagnosed up to 8 years later than the primary lesion. These lesions are usually multiple and most commonly located in frontoparietal regions of the brain. Previous reports have shown interleukin-6 (IL-6) to be involved in the induction of intracellular adhesion molecule-1 (ICAM-1) during the period of embolization and metastasis (7). The mechanism of tumor metastases has not been fully established. However, it has been postulated that tumor tissues may grow into the walls of the vessels causing focal disruption of the internal elastic lamina, providing a nidus for cerebral hemorrhage and subsequent growth of metastatic tumor tissue (8). It is most likely, the intracerebral hemorrhagic metastases resulted from silent emboli of the cardiac myxoma occuring in the pre-operative period or due to operative manipulation. These myxomatous emboli survive in the vessels for varying unpredictable durations and later cause focal disruption of internal elastic lamina leading to hemorrhage or aneurysmal formation in rare cases. These findings imply that a non-malignant myxoma is capable of metastatic spread despite its slow growth and innocuous histological appearance. The imaging findings of cerebral brain metastasis from cardiac myxoma correlate with the tumor metastatic mechanism. The typical angiographic features of myxoma-associated aneurysms are well described as fusiform outpouchings or saccular anerusyms (9). CT morphology of cerebral myxoma metastasis usually appears as a hyperdense lesion with some contrast enhancement due to extensive hemorrhage (10). MR images show peripheral dark signal intensity rim on T1 and T2-weighted images which can be explained by dense accumulation of iron and hemosiderin from chronic recurrent hemorrhage, and occasionally superfical siderosis. The multiplicity of the lesion, the typical imaging findings of hemorrhage and enhancement of the cerebral lesions in patients with completely resected cardiac myxomas such as our case can suggest a diagnosis of cerebral metastasis. Treatment has not been well-established. Surgery is usually performed in cases having one or two metastatic lesions showing favorable prognosis. For multiple lesions, whole brain radiotherapy with 50 Gy doses has been preferred in reported literature. However, there have been only a few reports on the outcome of whole brain radiotherapy and further study would be necessary to propose a treatment guideline for these patients.
In summary, cardiac myxomas show good prognosis when completely resected. However we report here a rare case of delayed cerebral metastases from completely resected cardiac myxoma. Periodic long-term follow-up evaluation including brain imaging would be helpful for early detection of both recurrence and metastasis.
Figures and Tables
Fig. 1
A 49-year-old female patient underwent resection of the cardiac myxoma that caused multiple cerebral embolic infarction. Photomicrograph of the histopathologic specimen shows spindled and stellite cells with eosinophilic cytoplasm and round to slender nucleus embedded in pale edematous myxoid matrix. No significant cytologic atypia including marked pleomorphism and abnormal mitotic figures was seen (H-E, ×400).
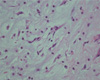
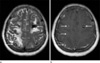
Fig. 2
Patient unerwent MRI due to symptoms of sudden dysarthria 1-year after resection of cardiac myxoma.
(a) T2-weighted image shows multiple peripheral dark signal intensity rims in bilateral cerebral hemispheres, and adjacent superficial siderosis, suggestive of hemorrhagic metastases. (b) T1-post contrast image shows enhancement of the metastatic lesions.
References
1. Amano J, Kono T, Wada Y, et al. Cardiac myxoma: its origin and tumor characteristics. Ann Thorac Cardiovasc Surg. 2003. 9:215–221.
2. Stratakis CA, Kirschner LS, Carney JA. Carney complex: diagnosis and management of the complex of spotty skin pigmentation, myxomas, endocrine overactivity, and schwannomas. Am J Med Genet. 1998. 80:183–185.
3. Pinede L, Duhaut P, Loire R. Clinical presentation of left atrial cardiac myxoma. A series of 112 consecutive cases. Medicine (Baltimore). 2001. 80:159–172.
4. Rodriguez FJ, Brown RD, Mohr JP, et al. Embolic atrial myxoma with neoplastic aneurysm formation and haemorrhage: a diagnostic challenge. Neuropathol Appl Neurobiol. 2006. 32:213–216.
5. Altundag MB, Ertas G, Ucer AR, et al. Brain metastasis of cardiac myxoma: case report and review of the literature. J Neurooncol. 2005. 75:181–184.
6. McCarthy PM, Piehler JM, Schaff HV, et al. The significance of multiple, recurrent, and "complex" cardiac myxomas. J Thorac Cardiovasc Surg. 1986. 91:389–396.
7. Wada A, Kanda T, Hayashi R, Imai S, Suzuki T, Murata K. Cardiac myxoma metastasized to the brain: potential role of endogenous interleukin-6. Cardiology. 1993. 83:208–211.
8. Ng HK, Poon WS. Cardiac myxoma metastasizing to the brain. Case report. J Neurosurg. 1990. 72:295–298.
9. Hwang BJ, Connelly MM, Lev MH. Distinctive MR imaging appearance of hemorrhagic cerebral aneurysms associated with atrial myxoma. AJR Am J Roentgenol. 2001. 177:925–927.
10. Hofmann E, Becker T, Romberg-Hahnloser R, Reichmann H, Warmuth-Metz M, Nadjmi M. Cranial MRI and CT in patients with left atrial myxoma. Neuroradiology. 1992. 34:57–61.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download