Abstract
Squamous cell carcinoma of the pancreas is a rare, uncommon tumor that is characterized by squamous metaplasia of the ductal columnar cells. We report the image findings of a rare case of the pancreatic squamous cell carcinoma associated with chronic pancreatitis.
Squamous cell carcinoma of the pancreas is very rare, and few case reports have been published (1, 2). Although squamous cells are not normally present in the pancreas, the inflammatory episodes such as pancreatitis may cause squamous metaplasia of the ductal columnar cells (2-5). Squamous metaplasia of pancreatic duct is found in 9-64% of pancrease routinely examined at autopsy, but transformation into squamous cell carcinoma is extremely rare (5). This is the first report in English literature that describes the MR imaging findings of squamous cell carcinoma of the pancreas. In this report, we review our experience with primary squamous cell carcinoma of the pancreas in patient with chronic pancreatits, with emphasis on its unique imaging features which distinguish this entity from complication of chronic pancreatitis or other pancreatic cancer.
A 61-year-old man was admitted to our hospital with a five-month history of chronic, intermittent epigastric pain, and an unintentional weight loss of 10 kg. He had a medical history of 10 years of diabetes mellitus. Physical examination on admission revealed mild tenderness over the epigastrium. Laboratory investigation revealed elevations in serum glucose 446 mg/dL (reference: 70-100 mg/dL), alkaline phosphatase 381 U/L (normal: 103-335 U/L), carcinoembryonic antigen 7.9 ng/mL (reference: 0-6 ng/mL), c-reactive protein 1.69 mg/dL (reference: 0-0.3 mg/dL ), erythrocyst sedimentation rate 23 mm/hr (reference:1-22 mm/hr), and serum amylase 165 IU/L (reference: 43-116 IU/L). Blood cell count, hemoglobin, total bilirubin, serum lipase, aspartate aminotransferase, alanine aminotransferase, and CA19-9 levels were within normal range.
Plane abdominal radiograph revealed multiple calcifications and stones in epigastric area. Contrast-enhanced computed tomography (CT) demonstrated pancreatic parenchymal atrophy with multiple calcifications and ductal stones, and 4×3×4.2 cm heterogeneously low attenuated periduatal mass with delayed peripheral enhancement in pancreatic tail, and directly invaded the adjacent splenic hilum (Fig. 1a). Peripheral and central enhancement of the pancreatic mass was measured as 40-44 HU on non-enhanced and arterial phase, 50 HU and 57 HU on portal phase, 55 HU and 88 HU on equilibrium phase, respectively. Endoscopic ultrasound (EUS) showed iso-echogenic solid mass with multiple ductal stones and parenchymal calcifications in pancreas (Fig. 1b). EUS-guided needle biopsy was performed. MR images showed well defined pancreatic tail mass with iso-signal intensity on T1-weighted image (Fig. 1c), and inhomogeneously high signal intensity on T2-weighted image (Fig. 1d). EUS guided biopsy revealed well-differentiated squamous cell carcinoma of pancreas.
The patient underwent a partial pancreatectomy, gastric wedge resection, and a splenectomy. On gross pathologicspecimen, 4.9×4.5×4.0 cm solid tumor encircles main duct containing stone, and extended to peripancreatic tissue, gastric mucosal wall, splenic hilum (Fig. 1e). Microscopic examination demonstrated lymphovascular, perineural and perigastric lymph node invasion. Histologic examination demonstrated squamous cell carcinoma with intraparenchymal tumor nest, keratin pearl, and clear cell change (Fig. 1f). The patient's post-operative course was uneventful. After 1 month, concurrent chemoradiotheraphy was performed.
Squamous cell carcinoma (SCC) of pancreas is very rare, and the histogenesis of squamous cell carcinoma of the pancreas is uncertain. It is speculated to have originated from a primitive cell capable of differentiating into either squamous or glandular carcinoma, adenocarcinoma undergoing squamous transformation, aberrant squamous cell undergoing a malignant change, or malignant transformation of the squamous metaplasia of the ductal epithelium (5-7). Etiologic factors of squamous metaplasia of ductal epithelium are reported to include chronic inflammation, old age, and vitamin A deficiency (2). It is not uncommon to find squamous metaplasia of the ductal columnar cells during the period of inflammation, such as in pancreatitis (1). This hypothesis is strengthened by the SCC arising in periductal area in our case with chronic pancreatitis.
Contrast enhanced CT findings of SCC reported an increase from 35 Hounsfield units (HU) to a maximum of 61 HU over 6 minutes (8). On delayed enhancing CT scan, peripheral portion of SCC was demonstrated (from 40 HU to 88 HU), but central portion of the SCC demonstrated lower enhancement (from 40 HU to 55 HU). This finding can be correlated with peripheral enhancing pattern of tumor blush or hypervascularity on angiography. Angiographic finding of SCC was reported as tumor blush pattern with new vessel formation (9, 10). MRI showed well-defined solid mass with iso-signal intensity on T1WI, and heterogeneously high signal intensity on T2WI. There was no cystic necrosis or hemorrhage in heterogeneous solid mass on MR images. To our knowledge, there was no report of MRI findings of pancreatic squamous cell carcinoma.
The differential diagnosis of SCC of pancreas should be considered such as solitary papillary tumor (SPT), adenocarcinoma, and endocrine tumors. SPT lesions usually are large and encapsulated, frequently contain varying amounts of necrosis, hemorrhage, cystic changes, but do not contain pancreatic ductal dilatation, pancreatic atrophy while accompanied by a very high signal intensity on fat saturated T2-weighted images, all of which are known to be the characteristic findings of solid pseudopapillary tumors that allow differentiation from other solid pancreatic tumors (11). An ill-defined tumor margin and the presence of pancreatic ductal dilatation and atrophy strongly favored adenocarcinoma (11). Moreover, peripheral enhancement with new vessel formation or tumor blush pattern of SCC is uncommon in adenocarcinoma. Pancreatic leiomyosarcoma was reported as a well circumscribed mass with diffusely homogeneously or heterogeneously solid lesion with cystic tumor necrosis (12). However, these nonspecific image findings are also detected in fibrosarcoma, malignant fibrohystiocytoma, liposarcoma, rhabdomyosarcoma and malignant hemangiopericystoma (9). Early homogeneous and persistent enhancement favored small sized endocrine tumor with increasing size, and solid. In addition ,well demarcated endocrine tumors tend to appear cystic due to central necrosis and hemorrhage (11).
In our case, the features of squamous cell carcinoma that enabled differentiation from complication of chronic pancreatitis, periductal location, well-defined tumor margin, delayed perhpheral enhancement on CT scan and heterogeneously high signal intensity on T2WI. In spite of a well-defined tumor margin, the perilesional irregular fatty infiltration can be suspected malignant invasion to the adjacent organs. PET scan, FDG uptake were useful findings for diagnosis of malignant lesion.
The clinical presentation of pancreatic squamous cell carcinoma is vague and indistinguishable from that of adenocarcinoma, with the most common presenting symptoms being abdominal pain, weight loss, nausea, vomiting and obstructive jaundice (10,13). Although laboratory investigation is not helpful in the diagnosis of squamous cell carcinoma of pancreas, elevation of squamous cell carcinoma antigen and serum calcium was reported in patients of pancreatic squamous cell carcinoma (10,13). SCC antigen may be a useful marker for tumor recurrence, but the association requires further validation, and hypercalemia is thought to be mediated through various humoral mechanisms (1, 13).
The biologic behavior of pancreatic SCC appears to be similar to that of the ductal adenocarcinoma. Both tend to occur in older people, and are usually metastatic at the time of diagnosis, respond poorly to chemotheraphy and radiotheraphy (1). The literature on squamous cell carcinoma of pancreas has reported a variety of median survivals with one study showing a median survival of 7 months for patients who underwent curative resection and 3 months for patients who did not undergo curative resection (4). On study of adenosquamous cell carcinoma of pancreas, squamous cell carcinoma was associated with a better prognosis with late metastasiswith adenocarcinomatous elements possessing more malignant potential than squamous elements (6). No criteria exist for the cytological distinction of metastatic from primary pancreatic SCC, and this situation again emphasizes the importance of searching for another primary source (1).
In conclusion, SCC is a rare pancreatic malignant lesion; the differential diagnosis was difficult. In our case, SCC images included concomitant chronic pancreatitis, periductal location, delayed peripheral enhancement on CT scan, iso-intense on T1WI, heterogeneously hyperintense on T2WI, and positively on FDG-PET. These findings are considered as useful image findings for the diagnosis of SCC.
Figures and Tables
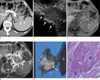 | Fig. 1(a) Contrast enhanced CT scan shows heterogeneously peripheral enhancing solid mass (arrowheads) and perilesional invasion (white arrows) on equilibrium phase. Note dense calcifications (black arrow) within diffuse atrophic change of pancreas, suggesting chronic pancreatitis. (b) EUS (endoscopic ultrasonography) shows lobulating solid mass (arrowheads) in tail portion of pancreas, and multiple hyperechogeic stones (arrows) in tail of pancreas. (c) Axial T1-weighted MR image shows iso-signal intensity of the pancreatic mass lesion (arrow heads) than that of spleen. (d) Axial T2-weighted MR image shows heterogeneously hyper intense mass (arrow heads) in pancreatic tail and perilesional infiltration in splenic hilum (white arrows). Signal intensity of mass lesion is higher than that of spleen. Intraductal stone (black arrow) and parenchyma atrophy of pancreas are suggestive findings of chronic pancreatitis. (e) Surgical specimen shows solid mass (arrowheads) in distal pancreas extends to the peripancreatic soft tissue and hilum of spleen (S). Note the small intraductal and stones (arrow). (f) Histologic section demonstrating a squamous cell carcinoma with intraparenchymal tumor nest (arrow heads), keratin pearl (white arrow), and clear cell change (black arrow) (H & E stain, 200 × magnification). |
References
1. Al-Sherhri A, Silverman S, King KM. Squamous cell carcinoma of the pancreas. Current Oncology. 2008. 15:293–297.
2. Lowry CC, Whitaker HW Jr, Greiner DJ. Squamous cell carcinoma of the pancreas. South Med J. 1949. 42:753–757.
3. Mulkeen AL, Yoo PS, Cha C. Less common neoplasms of the pancreas. World J Gastroenterol. 2006. 12(20):3180–3185.
4. Brown HA, Dotto J, Robert M, Salem RR. Squamous cell carcinoma of the pancreas. J Clin Gastroenterol. 2005. 39:915–919.
5. Anagnostopoulos GK, Aithal GP, Ragunath K, Kaye P, Rowlands BJ. Squamous cell carcinoma of the pancreas: report of a case and review of the literature. JOP. 2006. 7:47–50.
6. Ishikawa O, Matsui Y, Aoki I, Iwanaga T, Terasawa T, Wada A. Adenosquamous carcinoma of the pancreas: a clinicopathologic study and report of three cases. Cancer. 1980. 46:1192–1196.
7. Nakashima H, Hayakawa T, Hoshino M, et al. Squamous cell carcinoma of the pancreas with massive invasion of the retroperitoneum. Intern Med. 1995. 34:61–64.
8. Fajardo LL, Yoshino MT, Chernin MM. Computed tomography findings in squamous cell carcinoma of the pancreas. J Comput Assist Tomogr. 1988. 12:138–139.
9. Sprayregen S, Schoenbaum SW, Messinger NH. Angiographic features of squamous cell carcinoma of the pancreas. J Can Assoc Radiol. 1975. 26:122–124.
10. Minami T, Fukui K, Morita Y, et al. A case of squamous cell carcinoma of the pancreas with an initial symptom of tarry stool. J Gastroenterol Hepatol. 2001. 16:1077–1079.
11. Yu MH, Lee JY, Kim MA, et al. MR imaging features of small solid pseudopapillary tumors: retrospective differentiation from other small solid pancreatic tumors. AJR Am J Roentgenol. 2010. 195:1324–1332.
12. Paciorek ML, Ross GJ. MR imaging of primary pancreatic leiomyosarcoma. Br J Radiol. 1998. 71:561–563.
13. Brayko CM, Doll DC. Squamous cell carcinoma of the pancreas associated with hypercalcemia. Gastroenterology. 1982. 83:1297–1299.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download