Abstract
Purpose
To assess the frequency and severity of acute adverse reactions to intravenous administration of gadolinium-based contrast agents using computerized reporting system at a single large academic institution.
Materials and Methods
We assessed data from electronic hospital information system from October 2008 to December 2010. Reactions were classified as mild, moderate, or severe. We compared the frequency of adverse reactions among three contrast agents (Gd-BT-DO3A, Gd-DTPA and Gd-EOB-DTPA).
Results
The total number of administrated contrast agents was 33,600, and the number of administration of Gd-BT-DO3A, Gd-DTPA and Gd-EOB-DTPA were 20,824 (62%), 10,417 (31%) and 2,359 (7%), respectively. Total 39 adverse reactions were reported accounting for 0.1161% of all administrations. The incidences of adverse reactions were 0.1248% (26/39, 67%) for Gd-BT-DO3A, 0.0768% (8/39, 21%) for Gd-DTPA, and 0.2120% (5/39, 13%) for Gd-EOB-DTPA. The difference of frequencies of adverse reaction among three contrast agents was not significant. Most cases of the adverse effect were mild (35/39, 89.7%). Moderate and severe adverse reactions were encountered in two patients, respectively.
Figures and Tables
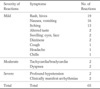
Fig. 1
Distributions of severity of adverse reactions by contrast agents.
(a) Overall distribution of severity of adverse reactions by all three contrast agents. (b) Distribution of severity of adverse reactions by Gd-BT-DO3A. (c) Distribution of severity of adverse reactions by Gd-DTPA. (d) Distribution of severity of adverse reactions by Gd-EOB-DTPA.

References
1. Niendorf HP, Dinger JC, Haustein J, Cornelius I, Alhassan A, Clauss W. Tolerance data of Gd-DTPA: a review. Eur J Radiol. 1991. 13:15–20.
2. Nelson KL, Gifford LM, Lauber-Huber C, Gross CA, Lasser TA. Clinical safety of gadopentetate dimeglumine. Radiology. 1995. 196:439–443.
3. Murphy KJ, Brunberg JA, Cohan RH. Adverse reactions to gadolinium contrast media: a review of 36 cases. AJR Am J Roentgenol. 1996. 167:847–849.
4. Li A, Wong CS, Wong MK, Lee CM, Au Yeung MC. Acute adverse reactions to magnetic resonance contrast media--gadolinium chelates. Br J Radiol. 2006. 79:368–371.
5. Dillman JR, Ellis JH, Cohan RH, Strouse PJ, Jan SC. Frequency and severity of acute allergic-like reactions to gadolinium-containing iv contrast media in children and adults. AJR Am J Roentgenol. 2007. 189:1533–1538.
6. Bleicher AG, Kanal E. Assessment of adverse reaction rates to a newly approved MRI contrast agent: review of 23,553 administrations of gadobenate dimeglumine. AJR Am J Roentgenol. 2008. 191:W307–W311.
7. Marckmann P, Skov L, Rossen K, et al. Nephrogenic systemic fibrosis: suspected causative role of gadodiamide used for contrast-enhanced magnetic resonance imaging. J Am Soc Nephrol. 2006. 17:2359–2362.
8. Manual on contrast media version 7. Tne American College of Radiology. 2010. www.acr.org/SecondaryMainMenuCategories/quality_safety/contrast_manual/FullManual.aspx.
9. Sadowski EA, Bennett LK, Chan MR, et al. Nephrogenic systemic fibrosis: risk factors and incidence estimation. Radiology. 2007. 243:148–157.
10. Shellock FG, Parker JR, Pirovano G, et al. Safety characteristics of gadobenate dimeglumine: clinical experience from intraand interindividual comparison studies with gadopentetate dimeglumine. J Magn Reson Imaging. 2006. 24:1378–1385.
11. Murphy KP, Szopinski KT, Cohan RH, Mermillord B, Ellis JH. Occurrence of adverse reactions to gadolinium-based contrast material and management of patients at increased risk: a survey of the American Society of Neuroradiology Fellowship Directors. Acad Radiol. 1999. 6:656–664.
12. Knopp MV, Balzer T, Esser M, Kashanian FK, Paul P, Niendorf HP. Assessment of utilization and pharmacovigilance based on spontaneous adverse event reporting of gadopentetate dimeglumine as a magnetic resonance contrast agent after 45 million administrations and 15 years of clinical use. Invest Radiol. 2006. 41:491–499.
13. Abujudeh HH, Kosaraju VK, Kaewlai R. Acute adverse reactions to gadopentetate dimeglumine and gadobenate dimeglumine: experience with 32,659 injections. AJR Am J Roentgenol. 2010. 194:430–434.
14. Morgan DE, Spann JS, Lockhart ME, Winningham B, Bolus DN. Assessment of adverse reaction rates during gadoteridolenhanced MR imaging in 28,078 patients. Radiology. 2011. 259:109–116.
15. Forsting M, Palkowitsch P. Prevalence of acute adverse reactions to gadobutrol--a highly concentrated macrocyclic gadolinium chelate: review of 14,299 patients from observational trials. Eur J Radiol. 2010. 74:e186–e192.
16. Bluemke DA, Sahani D, Amendola M, et al. Efficacy and safety of MR imaging with liver-specific contrast agent: U.S. multicenter phase III study. Radiology. 2005. 237:89–98.
17. Lalli AF. Contrast media reactions: data analysis and hypothesis. Radiology. 1980. 134:1–12.
18. Gonen M, Panageas KS, Larson SM. Statistical issues in analysis of diagnostic imaging experiments with multiple observations per patient. Radiology. 2001. 221:763–767.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download