Abstract
Purpose
To retrospectively evaluate the usefulness of diffusion-weighted images, ADC maps and conventional MR images for determination of brain tumor consistency.
Materials and Methods
Twenty-three patients with brain tumor underwent MR examinations with T1, T2 and diffusion-weighted images. Regions of interest (ROIs) were drawn in the tumors, and the measured signal intensities (SI) were normalized with the contralateral side. We evaluated the correlation between SI ratios from various images and tumor consistency assessed at surgery. In three patients with both cystic and solid components, each component was evaluated independently. Qualitatively observed SIs were also correlated with tumor consistency.
Results
Statistical analysis revealed significant correlation between tumor consistency and ADC ratio (r = -0.586, p = 0.002), SI ratios on T2-weighted images (r = -0.497, p = 0.010), and observed SIs on T2-weighted images (r = -0.461, p = 0.018). The relative ratio of ADC value correlated with tumor consistency most strongly.
The surgical management of brain tumor is the first step of treatment. Preoperative planning for excision of intra-axial brain tumor requires knowledge about tumor consistency as well as location, size and invasiveness. Operating time is also affected by tumor consistency; whereas soft tumors can easily removed with suction, careful and diligent dissection is required for harder tumors. Thus information of the consistency of intra-axial brain tumor may help the clinician plan the proper surgical technique before the procedure.
Several earlier studies assessed the ability of conventional MR imaging for determining the consistency of pituitary adenomas (1-6) or meningiomas (7-11). Only a few studies evaluated the consistency of pituitary macroadenomas using apparent diffusion coefficient (ADC) with diffusion weighted imaging (DWI) (12-14). To the best of the authors' knowledge, a detailed analysis of DWI and conventional MR imaging for prediction of consistency of the intra-axial brain tumor has not yet been reported. Therefore, the aim of this study was to evaluate the usefulness of DWI, ADC maps and conventional MR images for determination of intra-axial brain tumor consistency.
We evaluated the MR images of 23 patients (10 men, 13 women; mean age, 52 years ± 18.20) with intra-axial brain tumors, who had undergone MR examinations between 2004 and 2008. Histologic types were glioblastoma multiforme (n = 12), metastasis (n = 4), anaplastic oligodendroglioma (n = 2), pilocystic astrocystoma (n = 2), anaplastic oligoastrocytoma (n = 1), anaplastic astrocytoma (n = 1), atypical teratoid/rhabdoid tumor (n = 1).
MR images were acquired using 1.5T MR scanner (Signa Excite, GE Medical Systems, Milwaukee, WI, U.S.A. or Achieva, Philips Medical Systems, Best, Netherlands) with a 8-channel head coil. The conventional MR images and DW images were obtained separately. The interval between two studies was shorter than 1 week. The axial spin echo T1-weighted image (T1WI) (repetition time (TR), 500-650 ms; echo time (TE), 12-13 ms; section thickness, 5 mm; field of view (FOV), 220 × 220 mm; matrix size, 256-320 × 224; number of excitation (NEX), 1) and the axial turbo spin echo T2-weighted image(T2WI) (TR, 3600-4500 ms; TE, 80-122 ms; Echo train length(ETL), 12-16; section thickness, 5 mm; FOV, 220 × 220 mm; matrix size, 256-320 × 220-256; NEX, 1-2) were used for the signal intensity(SI) measurement of the tumors.
DWI was performed using a single-shot spin-echo echo-planar imaging sequence (TR, 2330-2640 ms; TE, 65-89 ms; flip angle, 90° section thickness, 5 mm; FOV, 230 × 230 mm; matrix size, 128 × 128; b-values of 0 and 1000 s/mm2).
Measurement of SI of the tumors on T1WI, T2WI, DWI and ADC maps was performed by placing regions of interest (ROIs) drawn in the tumors. The measured SIs of the tumors were normalized by dividing the tumor SI values by the SIs of the contralateral normal regions. In three patients with both cystic and solid components, each component was evaluated independently.
Qualitative observed SIs by two experienced neuroradiologists were graded into 7 steps (grade 3 to grade -3) on various images : grade 3, marked hyperintense; grade 2, moderate hyperintense; grade 1, mild hyperintense; grade 0, isointense relative to the normal brain parenchyma; grade -1, mild hypointense; grade -2, moderate hypointense; grade -3, black signal intensity.
All patients with intra-axial brain tumor underwent surgical resection performed by one neurosurgeon. 16 tumors were totally resected and 7 tumors were partially removed. At surgery, consistency of the tumors was classified ac cystic, gelatinous, friable, soft, firm or hard.
Statistical analysis was performed by using the Statistical Package for the Social Sciences. We used analysis of variance to assess the difference among the relative SI ratios on various MR images across tumor consistency group, followed by a post hoc comparison (Tukey's honest significance test). The Kruskal-Wallis test was used to evaluate differences in qualitative observed SI grade on various MR images among all consistency groups. We evaluated the correlation between relative SI ratios and qualitatively observed SI grade from various MR images and tumor consistency assessed at surgery by using Pearson's and Spearman's correlation coefficient. A p value of less than 0.05 was considered a statistically significant difference.
The table 1 and 2 summarize the relationship between MR imaging findings and consistency of intra-axial brain tumors. No correlation was found between tumor consistency and relative SI ratio on T1WI. Statistical analysis also revealed no significant correlation between tumor consistency and qualitative SI grade on T1WI.
Representative conventional MR images and ADC maps are shown in Figs. 1, 2, 3, 4. The mean ratio of ADC in the cystic consistency group was 3.46 ± 0.55, 2.75 ± 0.29 in the gelatinous group, 1.84 ± 0.78 in the friable group, 1.87 ± 0.62 in the soft group, 1.82 ± 0.67 in the firm group, and 1.63 ± 0.10 in the hard group. The difference in the mean ratio of ADC values among various consistency groups was statistically significant (p = 0.012). Pearson's correlation analysis also demonstrated that relative SI ratios of ADC was a significant predictor of tumor consistency (r = -0.586, p = 0.002) (Fig. 5). Cystic consistency tumor was associated with a significantly higher relative SI ratio than friable, soft, firm and hard groups (Tukey HSD p < 0.05).
The mean SI ratio on T2WI in the cystic consistency group was 2.73 ± 0.94, 1.96 ± 0.39 in the gelatinous group, 1.68 ± 0.20 in the friable group, 1.78 ± 0.38 in the soft group, 1.81 ± 0.37 in the firm group, and 1.44 ± 0.52 in the hard group with a statistically significant difference (p = 0.037). Significant correlation was found between relative SI ratio on T2WI and tumor consistency(r = -0.497, p = 0.01) (Fig. 6). Cystic tumor was associated with significantly higher SI ratio than hard tumor (Tukey HSD p < 0.05). The SI ratio on T1WI showed no significant difference among various consistency groups.
The qualitatively observed SI on T2WI in the cystic consistency group was 2.67 ± 0.58, 2.50 ± 0.71 in the gelatinous group, 1.50 ± 0.55 in the friable group, 1.22 ± 0.67 in the soft group, 1.75 ± 0.50 in the firm group, and 1.0 ± 0.0 in the hard group with a statistically significant difference (p = 0.035) (Fig. 7). Spearman's correlation analysis showed significant correlation between qualitatively observed SI grade and tumor consistency (r = -0.461, p = 0.018). Kruskal-Wallis test indicated that there was a significant difference between observed SI grade on ADC and DWI for consistency groups (p = 0.031, 0.039, respectively). But there was no significant correlation between tumor consistency and SI grade on ADC and DWI (p = 0.059, 0.058, respectively).
Several reports assessed the ability of conventional MR images and DWI to predict consistency of pituitary adenomas or meningiomas and their results varied (12-14). Our study is the first research that evaluated the consistency of intra-axial brain tumors by various MR images. We found significant correlation between relative SI ratios of tumors on T2WI and qualitatively observed SI grade on T2WI and tumor consistency. In the present study, softer tumors showed higher T2 SI and grade than harder tumors did. Pierallini et al. (12) described inverse relation between SI on T2WI and tumor consistency of pituitary macroadenoma. Other researchers reported no correlation between tumor consistency and SI on conventional MR images in pituitary adenomas and meningiomas (4-6, 11). Such divergent findings may result from different tissue component of tumors. Collagen content, cellularity, extracellular space and free water contents are strongly correlated with tumor consistency. Harder tumor has more collagen content, higher cellularity, lower extracellular space and free water content than softer tumor does. Whereas extracellular space and free water contents produce an increase in SI on T2WI, fibrous tissue and high cellularity cause a decrease in SI. Therefore harder tumor demonstrates lower SI on T2WI than softer tumor does.
We found no statistically significant correlation between tumor consistency and relative SI ratio on T1WI and qualitatively observed SI grade on T1WI. Our results agreed with those in previous studies that showed no significant difference in SI on T1WI among tumor consistency groups (12, 13). Fibrous tissue which is abundant in harder tumor decrease SI on T1WI and extracellular space and free water contents which are rich in softer tumor also produce a decrease in SI on T1WI. Therefore, T1WI is of limited value in the evaluation of tumor consistency.
In this study, relative ratios of ADC of harder tumors were significantly lower than those of softer tumors. Pearson's correlation analysis also demonstrated that relative ratios of ADC were significant predictors of tumor consistency. More collagen content, higher cellularity and lower extracellular space may inhibit diffusion, resulting in a lower ADC (15, 16). In pituitary macroadenomas, a few reports evaluated the usefulness of DWI and ADC maps for determination of tumor consistency and their conclusions did not agree (12-14). Although line-scan diffusion-weighted imaging (LSDWI) (17) and periodically rotated overlapping parallel lines with enhanced reconstruction (PROPELLER) DWI (18) were less susceptible to artifact than echo-planar DWI, susceptibility artifact was inevitable particularly in pituitary region. Tumor consistency may also affected by necrosis, cystic degeneration and hemorrhage within tumors which may make tumor softer. In earlier studies, SI measurements were performed by placing ROIs only in solid portions of the tumors. However, we measured SI of the whole tumor. For ADC values, earlier researchers used absolute value of ADC for analysis. ADC values are affected by magnetic field inhomogeneity and other factors. So in this study, ADC values were normalized for interimager and interimage variability. They may make different result.
There were some limitations to this study. First, the sample size of 26 lesions is too small to confirm an exact relationship between tumor consistency and various MR images. Further studies with larger sample sizes are required to confirm these findings. Second, histologic analysis such as collagen content and quantitative estimation of fibrous tissue was not performed in this study. Third, some tumors were not totally removed. If totally removed, their consistencies may be rated differently. Lastly, intraoperative assessment of tumor consistency was qualitative. More precise quantification of tumor consistency may help in preoperative planning and we recommend that this should be performed in future studies.
In conclusion, the measured ratio of ADC, SI ratio and observed SI grade on T2WI can provide valuable information about the consistency of intra-axial brain tumor.
Figures and Tables
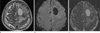 | Fig. 1Metastatic tumor with hard consistency in 44-year-old woman. (a) Axial T2WI shows a homogenous hyperintense (grade 1) mass. (b) Axial DWI shows a hypointense lesion (grade -2) with respect to normal white matter. (c) ADC maps shows a mass with increased diffusion coefficient (grade 1). |
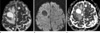 | Fig. 2Metastatic mucoepidermoid carcinoma with gelatinous consistency in 67-year-old man. (a) Axial T2WI shows homogenous hyperintense (grade 3) masses. (b) Axial DWI shows hypointense masses (grade -2) with respect to normal white matter. (c) ADC maps shows masses with increased diffusion coefficient (grade 3). |
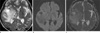 | Fig. 3Glioblastoma with friable consistency in 69-year-old man. (a) Axial T2WI shows a homogenous hyperintense (grade 2) mass. (b) Axial DWI shows a hypointense lesion (grade -1) with respect to normal white matter. (c) ADC maps shows a mass with slightly increased diffusion coefficient (grade 1). |
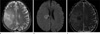 | Fig. 4Glioblastoma with friable consistency in 42-year-old man. (a) Axial T2WI shows a homogenous hyperintense (grade 1) mass. (b) Axial DWI shows a hyperintense lesion (grade 2) with respect to normal white matter. (c) ADC maps shows a mass with slightly decreased diffusion coefficient (grade -2). |
 | Fig. 5Box plot shows relative SI ratios on ADC in tumor consistency groups. Relative SI ratios on ADC are significantly correlated with intra-axial brain tumor consistency.
*, §, ¶ = significant different from cystic consistency group
*p = 0.02 , §p = 0.014, ¶p = 0.032
|
 | Fig. 6Box plot shows relative SI ratios on T2WI in tumor consistency groups. Relative SI ratios on T2WI are significantly correlated with intra-axial brain tumor consistency.
*, §, ¶ = significant different from cystic consistency group
*p = 0.033, §p = 0.044, ¶p = 0.045
|
 | Fig. 7Box plot shows qualitative SI grade on T2WI in tumor consistency groups. SI grade on T2WI are significantly correlated with intra-axial brain tumor consistency. |
References
1. Iuchi T, Saeki N, Tanaka M, Sunami K, Yamaura A. MRI prediction of fibrous pituitary adenomas. Acta Neurochir (Wien). 1998. 140:779–786.
2. Snow RB, Johnson CE, Morgello S, Lavyne MH, Patterson RH Jr. Is magnetic resonance imaging useful in guiding the operative approach to large pituitary tumors? Neurosurgery. 1990. 26:801–803.
3. Naganuma H, Satoh E, Nukui H. Technical considerations of transsphenoidal removal of fibrous pituitary adenomas and evaluation of collagen content and subtype in the adenomas. Neurol Med Chir (Tokyo). 2002. 42:202–212. discussion 213.
4. Bahuleyan B, Raghuram L, Rajshekhar V, Chacko AG. To assess the ability of MRI to predict consistency of pituitary macroadenomas. Br J Neurosurg. 2006. 20:324–326.
5. Chakrabortty S, Oi S, Yamaguchi M, Tamaki N, Matsumoto S. Growth hormone-producing pituitary adenomas: MR characteristics and pre- and postoperative evaluation. Neurol Med Chir (Tokyo). 1993. 33:81–85.
6. Hagiwara A, Inoue Y, Wakasa K, Haba T, Tashiro T, Miyamoto T. Comparison of growth hormone-producing and non-growth hormone-producing pituitary adenomas: imaging characteristics and pathologic correlation. Radiology. 2003. 228:533–538.
7. Yamaguchi N, Kawase T, Sagoh M, Ohira T, Shiga H, Toya S. Prediction of consistency of meningiomas with preoperative magnetic resonance imaging. Surg Neurol. 1997. 48:579–583.
8. Suzuki Y, Sugimoto T, Shibuya M, Sugita K, Patel SJ. Meningiomas: correlation between MRI characteristics and operative findings including consistency. Acta Neurochir (Wien). 1994. 129:39–46.
9. Maiuri F, Iaconetta G, de Divitiis O, Cirillo S, Di Salle F, De Caro ML. Intracranial meningiomas: correlations between MR imaging and histology. Eur J Radiol. 1999. 31:69–75.
10. Yrjana SK, Tuominen H, Karttunen A, Lahdesluoma N, Heikkinen E, Koivukangas J. Low-field MR imaging of meningiomas including dynamic contrast enhancement study: evaluation of surgical and histopathologic characteristics. AJNR Am J Neuroradiol. 2006. 27:2128–2134.
11. Kashimura H, Inoue T, Ogasawara K, et al. Prediction of meningioma consistency using fractional anisotropy value measured by magnetic resonance imaging. J Neurosurg. 2007. 107:784–787.
12. Pierallini A, Caramia F, Falcone C, et al. Pituitary macroadenomas: preoperative evaluation of consistency with diffusionweighted MR imaging--initial experience. Radiology. 2006. 239:223–231.
13. Suzuki C, Maeda M, Hori K, et al. Apparent diffusion coefficient of pituitary macroadenoma evaluated with line-scan diffusion-weighted imaging. J Neuroradiol. 2007. 34:228–235.
14. Mahmoud OM, Tominaga A, Amatya VJ, et al. Role of PROPELLER diffusion-weighted imaging and apparent diffusion coefficient in the evaluation of pituitary adenomas. Eur J Radiol. 2010. (Epub ahead of print).
15. Kono K, Inoue Y, Nakayama K, et al. The role of diffusionweighted imaging in patients with brain tumors. AJNR Am J Neuroradiol. 2001. 22:1081–1088.
16. Guo AC, Cummings TJ, Dash RC, Provenzale JM. Lymphomas and high-grade astrocytomas: comparison of water diffusibility and histologic characteristics. Radiology. 2002. 224:177–183.
17. Gudbjartsson H, Maier SE, Mulkern RV, Morocz IA, Patz S, Jolesz FA. Line scan diffusion imaging. Magn Reson Med. 1996. 36:509–519.
18. Pipe JG, Farthing VG, Forbes KP. Multishot diffusion-weighted FSE using PROPELLER MRI. Magn Reson Med. 2002. 47:42–52.




 PDF
PDF ePub
ePub Citation
Citation Print
Print




 XML Download
XML Download