Abstract
Purpose
To evaluate the magnetic resonance imaging (MRI) and clinicopathological features of triple negative breast cancer, and compare them with those of non-triple negative breast cancer.
Materials and Methods
This study included 231 pathologically confirmed breast cancers from January 2007 to May 2008. We retrospectively reviewed the MRI findings according to the Breast Imaging Reporting and Data System (BI-RADS) lexicon: mass or non-mass type, mass shape, mass margin, non-mass distribution, and enhancement pattern. Histologic type, histologic grade, and the results for epidermal growth factor receptor, p53, and Ki 67 were reviewed.
Results
Of 231 patients, 43(18.6%) were triple negative breast cancer. Forty triple negative breast cancers (93.0%) were mass-type lesion on MRI. A round or oval or lobular shape (p=0.006) and rim enhancement (p=0.004) were significantly more in triple negative breast cancer than non-triple negative breast cancer. In contrast, irregular shape (p=0.006) and spiculated margins (p=0.032) were significantly more in non-triple negative breast cancer. Old age (p=0.019), high histologic grade (p<0.0001), EGFR positivity (p<0.0001), p53 overexpression (p=0.038), and Ki 67 expression (<0.0001) were significantly associated with the triple negative breast cancer.
Triple negative (TN) breast cancer is a subtype that is negative for the three main receptors for breast cancer, namely estrogen receptor (ER), progesterone receptor (PR) and human epidermal growth factor receptor type 2 (HER2). TN breast cancer has been considered to have a clinical feature with aggressive behavior and poor prognosis because there is no specific therapeutic target for the tumor. Previous literatures reported that TN breast cancer had a pathologic entity with a high histologic grade, and an overexpression of molecular factors such as epidermal growth factor receptor (EGFR), and Ki 67 (1-4).
However, there are a few reports describing MR imaging features of TN breast cancer (5, 6). We hypothesized that comparison of MRI features between TN breast cancer and non-TN breast cancer could yield additional information for pretreatment planning and assessment of prognosis. Therefore, the purpose of our study was to evaluate the MRI findings of TN breast cancer and to compare the findings with those of non-TN breast cancer. We also compared the MRI findings with the clinicopathological results.
This study consisted of 302 consecutive patients who were confirmed to have breast cancer and who underwent breast MRI in our institution from January of 2007 to May of 2008. Seventy one patients were excluded from the analysis for the following reasons; 42 patients underwent MRI after the excision of breast cancer, 24 patients received neo-adjuvant chemotherapy, two patients had cancers that were not visible on MRI scans, one patient had recurrent breast cancer after surgery, one patient had breast sarcoma and one patient had no information available on the ER status of the breast cancer. Two hundred-thirty one breast cancers in 231 patients were enrolled in this study. In patients with two or more pathologically-confirmed tumors, including three patients with bilateral breast cancers, the largest one was selected.
Total 43 TN breast cancers and 188 non-TN breast cancers were included in this study. The age of the patients ranged from 31 to 86 years (mean age, 53.2 years). The size of the breast cancer ranged from 0.3 to 11.0 cm (mean size, 2.76 cm). This study was approved by the institutional review board of our institution.
MR images were acquired with a 1.5T scanner (Signa; GE Medical Systems, Milwaukee, WI, U.S.A. and Achieva; Philips Medical system, Best, the Netherlands) using a breast coil. MRI with the Signa scanner was performed using the following sequences; sagittal, fat-suppressed, fast spin-echo T2-weighted imaging, and axial or sagittal, fat-suppressed, fat-spoiled gradient-echo T1-weighted imaging (TR/TE=6.2/3.1, flip angle of 10°, 2.6 mm section thickness, and an acquisition time of 1 min 31 minutes) obtained before and 91, 182, 273, 364 and 455 sec after the rapid bolus injection of 0.2 mmol/kg body weight of Gd-DPTA (Magnevist, Schering, Berlin, Germany). MRI with the Achieva scanner was performed using the following sequences; sagittal, fat-suppressed, fast spin-echo T2-weighted imaging and axial fat-suppressed, fat-spoiled gradient-echo T1-weighted imaging obtained before and 91, 182, 273, 364 and 455 sec after the rapid bolus injection of the same contrast agent.
MRI findings for TN breast cancers and non-TN breast cancers were reviewed by the consensus of two radiologists (with 4 and 9 years of breast MR imaging experience). The morphology and enhancement of the lesions were described according to the BI-RADS lexicon. The lesions were divided into mass or non-mass types. Mass type lesions were assessed for size, shape, margins, and enhancement pattern. Non-mass type lesions were assessed for distribution and enhancement pattern. We evaluated the enhancement pattern on 2 min post-contrast MR images.
We reviewed the size, histologic type, and histologic grade of the TN and non-TN breast cancers. Immunohistochemistry was performed to assess the expression of the following molecular markers; ER, PR, HER2, p53, Ki 67, and epidermal growth factor receptor (EGFR). ER and PR positivity was defined as the presence of 10% or more positively stained nucleiin ten high-power fields. The intensity of HER2 membrane staining was scored as 0, 1+, 2+ or 3+. Tumors with 2+ or 3+ scores were classified as positive for HER2 overexpression, whereas tumors with scores of 0 or 1+ were negative for HER2 overexpression. Among 231 patients, assessment of EGFR was performed in 204 patients, p53 in 132 patients, and Ki 67 in 228 patients. EGFR was considered as positive if membrane staining was observed. Ki 67 expression level of >=15% was considered as expression.
Continuous variables are shown as means±standard deviation and categorical variables are presented as frequencies and percentages. The differences between the imaging findings for the TN cancer and non-TN cancer were compared using the unpaired t-test, Chi-square test and Fisher's exact test. In addition, logistic regression analysis was performed to assess the contribution of the major risk factors. Statistical significance was established at a p-value<0.05. Statistical analyses were performed using SAS version 9.1 (SAS Institute Inc., Cary, NC, USA).
Forty three patients (18.6%) had TN breast cancer, and 188 patients (81.4%) had non-TN breast cancer. The characteristics of TN breast cancer and non-TN breast cancer are summarized in Table 1. The mean age was significantly older for the TN cancer group compared with the non-TN cancer group (p=0.019). There was no significant difference in the tumor size between two groups.
TN breast cancers included invasive ductal carcinomas, medullary carcinomas, invasive lobular carcinomas, metaplastic carcinoma and ductal carcinoma in situ. Non-TN breast cancers included invasive ductal carcinoma, invasive lobular carcinoma, medullary carcinoma, mucinous carcinoma, papillary carcinoma, mixed carcinoma, metaplastic carcinoma and ductal carcinoma in situ. There were no significant differences in the histologic types between TN breast cancer and non-TN breast cancer groups.
TN breast cancers were more likely to have high histologic grade tumors (p<0.0001). For the TN breast cancers, 26 breast cancers (67%) were grade 3. For the non-TN breast cancers, 38 breast cancers (25%) were grade 3. Compared with non-TN breast cancer, TN breast cancer was associated with EGFR positivity (p<0.0001), p53 overexpression (p=0.038), and Ki 67 expression (p<0.0001).
Based on the multivariate analysis, a high histologic grade (odds ratio of grade 3 vs. grade 1, 9.1; 95% CI, 2.6-32.6), p53 overexpression (odds ratio, 2.5; 95% CI, 1.0-5.8), EGFR positivity (odds ratio, 5.2; 95% CI, 2.4-11.0), and Ki 67 expression (odds ratio of < 15% vs. >= 15%, 4.9; 95% CI, 2.3-10.5) were associated with the risk of TN breast cancer (Table 2).
TN breast cancers (93.0%) were more likely to show mass-type than non-TN breast cancers (80.8%). However, there was no significant difference in the lesion type between TN breast cancers and non-TN breast cancers (p=0.055).
Among mass-type lesions, 33 TN breast cancers (82.5%) showed round or oval or lobular shape. Irregular shape was found in 7 TN breast cancers (17.5%) and 69 non-TN breast cancers (45.4%). Irregular shape was significantly lesser in TN breast cancers than non-TN breast cancers (p=0.006) (Table 3). Smooth margins were more frequently found in TN breast cancers (37.5%) than non-TN breast cancers (23.0%). Spiculated margins were more frequently found in non-TN breast cancers (31.6%) than TN breast cancers (12.5%) (p=0.032). 25 TN breast cancers (62.5%) showed rim enhancement, which was significantly more than TN breast cancers (33.6%) (p=0.004). 59.9% of non-TN breast cancers showed heterogeneous enhancement (Fig. 1 and Fig. 2). There was no significant difference in the findings of non-mass lesions between TN breast cancers and non-TN breast cancers.
Based on the multivariate analysis, the oval, round and lobular shapes (hazard ratio 3.92; 95% CI, 1.6-9.4) and the rim enhancement (hazard ratio, 3.43; 95% CI, 1.6-7.3) were associated with the risk of TN breast cancer (Table 2).
Triple negative breast cancer has been reported in 10-25% of all types of breast cancer. It occurs with a higher incidence in pre-menopausal African/American women (1-4). In our study, TN breast cancer accounted for 18.6% of all types of breast cancers. Patients with TN breast cancer were older than those with non-TN breast cancer. This result was similar to the findings obtained for the Japanese series (2).
In our study, 93% of the TN breast cancers were mass-type lesions on MRI. Several studies have reported that TN breast cancer is more likely to exhibit mass on MRI and mammography (5-7). However, there was no significant difference in the lesion type between TN breast cancers and non-TN breast cancers in our study. This result may be due to the fact that 28 DCIS were included in our study. Non-mass type enhancement is a common feature of DCIS on MRI (8, 9).
We found that the lobular shape, smooth margins, and rim enhancement are associated with TN breast cancers. Because TN breast cancers have an aggressive growing nature, they may reveal bulging shape with pushing borders. Rim enhancement may be due to tumor necrosis. Uemastu T, et al. reported that a very high intratumoral signal intensity on T2-weighted MR images is associated with intratumoral necrosis (5). In contrast, non-TN breast cancers were associated with spiculated margins, due to the desmoplastic reaction of the tumor.
Metaplastic carcinoma and medullary carcinoma show a basal-like subtype, and they have higher incidences in TN breast cancer (10-15). Our study included five medullary carcinomas and one metaplastic carcinoma in 43 TN breast cancers. The frequency of the histologic types did not differ between the TN breast cancers and the non-TN breast cancers. This result may be due to the small number of TN breast cancers evaluated.
In our study, EGFR, p53 and Ki 67 were overexpressed in TN breast cancer. We assumed that aggressiveness and rapid growing of TN breast cancer related to overexpression of the markers. EGFR, a type of cell surface receptor, is associated with cell proliferation. EGFR has been considered as a potential therapeutic target in TN breast cancer (16). p53 is a tumor suppression gene that regulates cell proliferation and apoptosis (17). p53 overexpression is an indicator used to predict the response to anthracycline-based chemotherapy in breast cancer. They are associated with a poor prognosis (16-21). Ki 67 is a nuclear antigen that appears during the proliferative phase of the cell cycle. It is related to a high mitotic count and a high level of cell proliferation (22-24).
Our study has limitations. First, a small number of patients were enrolled. Further validation in a larger study is warranted. Second, we used only immunohistochemistry to define HER2 status. We classified the HER2 2+ score as positive for HER2 overexpression, without considering the results of fluorescence in situ hybridization (FISH). However, the definition of HER2 status in TN breast cancer remains controversial. Third, inter-observer variability in the assessment of BI-RADS-based MRI findings was not considered in the present study. However, two radiologists reached a consensus in the evaluation of the MRI findings.
In conclusion, TN breast cancer occurred in elderly women. On MRI, rim enhancing mass with round or oval or lobular shape is favorable to TN breast cancer rather than non-TN breast cancer. It may be due to aggressive histologic behavior of TN breast cancer. MRI finding may be helpful for planning treatment and prediction prognosis in triple negative and non-triple negative breast cancer patients.
Figures and Tables
Fig. 1
MRI finding for triple negative breast cancer in 42 year-old woman.
T1-weighted sagittal MR image with contrast enhancement shows a lobular mass with smooth margins and a rim enhancement pattern.
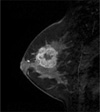
Fig. 2
MRI finding for non-triple negative breast cancer in 46 year-old woman.
T1-weighted sagittal MR image with contrast enhancement shows an irregular mass with spiculated margins and a heterogeneous enhancement pattern.
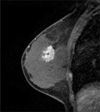
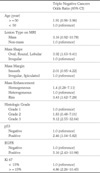
Table 1
Characteristics of the 43 Triple Negative Breast Cancer and 188 Non Triple Negative Breast Cancer Groups

References
1. Reis-Filho JS, Tutt AN. Triple negative tumours: a critical review. Histopathology. 2008. 52:108–118.
2. Iwase H, Kurebayashi J, Tsuda H, et al. Clinicopathological analyses of triple negative breast cancer using surveillance data from the Registration Committee of the Japanese Breast Cancer Society. Breast Cancer. 17:118–124.
3. Dent R, Trudeau M, Pritchard KI, et al. Triple-negative breast cancer: clinical features and patterns of recurrence. Clin Cancer Res. 2007. 13:4429–4434.
4. Trivers KF, Lund MJ, Porter PL, et al. The epidemiology of triple-negative breast cancer, includ ing race. Cancer Causes Control. 2009. 20:1071–1082.
5. Uematsu T, Kasami M, Yuen S. Triple-negative breast cancer: correlation between MR imaging and pathologic findings. Radiology. 2009. 250:638–647.
6. Chen JH, Agrawal G, Feig B, et al. Triple-negative breast cancer: MRI features in 29 patients. Ann Oncol. 2007. 18:2042–2043.
7. Yang WT, Dryden M, Broglio K, et al. Mammographic features of triple receptor-negative primary breast cancers in young premenopausal women. Breast Cancer Res Treat. 2008. 111:405–410.
8. Facius M, Renz DM, Neubauer H, et al. Characteristics of ductal carcinoma in situ in magnetic resonance imaging. Clin Imaging. 2007. 31:394–400.
9. Rosen EL, Smith-Foley SA, DeMartini WB, Eby PR, Peacock SLehman CD. BI-RADS MRI enhancement characteristics of ductal carcinoma in situ. Breast J. 2007. 13:545–550.
10. Weigelt B, Kreike B, Reis-Filho JS. Metaplastic breast carcinomas are basal-like breast cancers: a genomic profiling analysis. Breast Cancer Res Treat. 2009. 117:273–280.
11. Reis-Filho JS, Milanezi F, Steele D, et al. Metaplastic breast carcinomas are basal-like tumours. Histopathology. 2006. 49:10–21.
12. Leibl S, Moinfar F. Metaplastic breast carcinomas are negative for Her-2 but frequently express EGFR (Her-1): potential relevance to adjuvant treatment with EGFR tyrosine kinase inhibitors? J Clin Pathol. 2005. 58:700–704.
13. Reis-Filho JS, Milanezi F, Carvalho S, et al. Metaplastic breast carcinomas exhibit EGFR, but not HER2, gene amplification and overexpression: immunohistochemical and chromogenic in situ hybridization analysis. Breast Cancer Res. 2005. 7:R1028–R1035.
14. Kuroda N, Fujishima N, Inoue K, et al. Basal-like carcinoma of the breast: further evidence of the possibility that most metaplastic carcinomas may be actually basal-like carcinomas. Med Mol Morphol. 2008. 41:117–120.
15. Sasaki Y, Tsuda H. Clinicopathological characteristics of triple-negative breast cancers. Breast Cancer. 2009. 16:254–259.
16. Corkery B, Crown J, Clynes M, O'Donovan N. Epidermal growth factor receptor as a potential therapeutic target in triple-negative breast cancer. Ann Oncol. 2009. 20:862–867.
17. Rolland P, Spendlove I, Madjd Z, et al. The p53 positive Bcl-2 negative phenotype is an independent marker of prognosis in breast cancer. Int J Cancer. 2007. 120:1311–1317.
18. Viale G, Rotmensz N, Maisonneuve P, et al. Invasive ductal carcinoma of the breast with the "triple-negative" phenotype: prognostic implications of EGFR immunoreactivity. Breast Cancer Res Treat. 2009. 116:317–328.
19. Biswas DK, Iglehart JD. Linkage between EGFR family receptors and nuclear factor kappaB (NF-kappaB) signaling in breast cancer. J Cell Physiol. 2006. 209:645–652.
20. Nogi H, Kobayashi T, Suzuki M, et al. EGFR as paradoxical predictor of chemosensitivity and outcome among triple-negative breast cancer. Oncol Rep. 2009. 21:413–417.
21. Chae BJ, Bae JS, Lee A, et al. p53 as a specific prognostic factor in triple-negative breast cancer. Jpn J Clin Oncol. 2009. 39:217–224.
22. Yamamoto Y, Ibusuki M, Nakano M, Kawasoe T, Hiki R, Iwase H. Clinical significance of basal-like subtype in triple-negative breast cancer. Breast Cancer. 2009. 16:260–267.
23. Viale G, Regan MM, Mastropasqua MG, et al. Predictive value of tumor Ki-67 expression in two randomized trials of adjuvant chemoendocrine therapy for node-negative breast cancer. J Natl Cancer Inst. 2008. 100:207–212.
24. Ding SL, Sheu LF, Yu JC, et al. Expression of estrogen receptor-alpha and Ki67 in relation to pathological and molecular features in early-onset infiltrating ductal carcinoma. J Biomed Sci. 2004. 11:911–919.




 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download