Abstract
Brain aspergillosis has been increasing remarkably. They are known to occur commonly in immunocompromised individuals by hematogenous spread from other primary sites or by direct extension from adjacent structures to central nervous system. We report a rare case of a 29-year-old male without any known medical history, who had isolated brain lesion and the pathology from stereotactic biopsy confirmed cerebral aspergillosis.
Aspergillosis involving central nervous system (CNS) is known to occur frequently in the immunocom-promised individual owing to increased invasive feature of the disease and decreased host defense capacity(1, 2). The common pathway of CNS aspergillosis is hematogenous dissemination usually from lung or continuous extension from adjacent structures such as sinonasal cavity (3, 4). Therefore, the radiologic findings of isolated aspergillosis in immunocompetent patient have been rarely described in the published literature (5, 6).
We report an interesting case of a 29-year-old male without any known medical history who presented with fever and intractable headache. Isolated brain lesion was found in subsequent imaging studies which were pathologically confirmed as aspergillosis.
A 29-year-old man was referred presenting 15 days of high-grade fever, chills and intractable headache. The headache gradually aggravated with nausea and he experienced abnormal twitching in the left side arm and leg during the course of his illness. He did not have any significant past medical history and was not on any medication. There was no sign of infection in the middle auditory canals, oral cavity or nasal cavity on clinical examination. Total blood cell count and routine serum chemistry were all within normal range in peripheral blood including erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP).
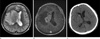
The patient underwent radiologic evaluation and the computerized tomography (CT) of the brain showed an ill-defined low density lesion at the right internal capsule and basal ganglia with mass effect to adjacent structures (Fig. 1A). Magnetic resonance imaging (MRI) of the brain revealed confluent multilobulating lesion in the right internal capsule and basal ganglia, which was hyperintense with hypointense peripheral rim on T2-weighted images (The lesion was hypointense without definite central and peripheral differentiation on T1-weighted images, which is not included in the figures). The lesion was located over the right basal ganglia and internal capsule infiltrating into adjacent corona radiata and thalamus with marked peripheral edema (Fig. 1b). Ill margined perilesional edema was depicted extending upward to right frontoparietal white matter and downward to pons. There were multifocal satellite lesions in the right frontal and parieto-occipital subcortical area (Fig. 1c, right frontal lesion is not depicted in the figures). On Gadolinium-enhanced MR T1-weighted images, strong enhancement along the margin of the lesion was noted with corresponding hypointense peripheral rim on T2-weighted images. On the other hand, central portion of the lesion with low signal intensity on T1-weighted images and high signal intensity on T2-weighted images, showed no enhancement (Fig. 1d). Restricted diffusion in the lesion center was suggested on the basis of internal hyperintensity on diffusion weighted images and low apparent diffusion coefficient (Fig. 1e). Stereotactic biopsy was done for pathologic confirmation and adequate treatment and it was reported as cerebral aspergillosis with characteristic fungal hyphae (Fig. 2). The patient's mental status aggravated after 3 weeks and brain MRI also showed compatible findings with aggravation of lesion in right basal ganglia with hydrocephalus (Fig. 3A, B). The mainstay management changed at this point from monotherapy to dual antifungal medication and the patient's clinical condition improved gradually afterwards. The follow up brain CT revealed concordant improvement of the lesion (Fig. 3c).
Aspergillosis is infection by ubiquitous fungus, Aspergillus. The most common pathogenic organism is A. fumigatus in the genus Aspergillus, but others such as A. flavus, A. Niger and A. oxyzae are also not hard to find (7). Aspergillosis involving CNS generally occurs by hematogenous spread from lung or less commonly by direct extension from paranasal sinuses, middle ear or oral cavity (4, 8).
There are rather many researches on CNS aspergillosis in immunocompromised patients, however, only a few reports were published on CNS aspergillosis in immunocompetent patient, especially on its radiologic findings, owing to its rareness (5-8). Furthermore, isolated CNS aspergillosis in healthy individual, like the presented case, with no other primary lesion draws attention for differential diagnosis because other entities like malignant metastasis, thromboembolic infarction or other abscesses could present overlapping image findings (3, 9, 10).
The imaging characters of CNS aspergillosis depend on host immune status and temporal stage of infection (2, 10, 11). Some investigators consider cerebritis and cerebral abscess are in continuum, cerebritis as early stage and the capsular form of abscess as the late stage. Ring enhancing capsule represents host immune reaction to isolate the infectious pathogen with liquefaction necrosis and purulent material containing center (4, 6).The presented case showed enhancing capsule with marked perilesional edema with corresponding hypersignal intensity on T2-weighted images with no enhancement. This observation could be explained by intact inflammatory process with lesional encapsulation and perilesional interstitial edema. The fact that most of CNS aspergillosis in immunocompromised patients showed minimal edema and contrast enhancements in other studies could be a good contrary proof and could be explained by lack of immune responses in these patients (3, 4).
Diffusion restriction feature of aspergillosis is thought to be due to intense cellular component and/or viscosity of inflammation, similar to that of pyogenic abscess (11). The low signal intensity rim of the lesion on T2-weight images could be related to presence of methemoglobin in the wall of the capsule, or to the free radicals produced by macrophages (3, 11).
Angioinvasive pathologic feature of CNS aspergillosis causes characteristic lesion distribution, which is involvement of small perforating arteries, such as basal ganglia, brain stem and corpus callosum. Its affinity for perforating arteries is explained by invasion within the walls for larger parent arteries to which aspergillus has spread hematogenously, subsequently compromising the origin of the perforating arteries (4, 7, 9). However, CNS aspergillosis also could be located in corticomedullary junction like any other septic embolic lesions or metastasis. In the presented case, the main lesion was located in the basal ganglia, thalamus, internal capsule and adjacent deep white matter as well as corticomedullary junction area for the satellite lesions.
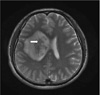
In the presented case, there was a hypointense rodlike structure within the lesion on T2-weight MR images (Fig. 4). This could be explained by the presence of dense aspergillus hyphal elements and paramagnetic elements such as iron and magnesium that is essential for hyphal growth. And this was also documented in previous literatures (7, 10). Absolute diagnosis of CNS aspergillosis can be made by confirmation of Aspergillus hyphae presence in brain tissue. The prognosis is known to be unfavorable for CNS aspergillosis, with the mortality rate of almost 100% in immunocompromised patients (2). However, early diagnosis and intense treatment with antifungal agents could amend better results in immunocompetent patients (2, 4, 6, 7).
In the presented case, it was initially difficult to tell the brain lesion was aspergillus abscess due to immunocompetent patient status and lack of other infectious origin. Awareness of various MR findings of brain abscess character according to host immune status, detection of fungal hyphal elements in the lesion which seem as rod-like tiny hypointensity lesion on T2-weighted images and knowing possibility of isolated brain aspergillosis in immunocompetent patient could improve prognosis in the future.
Figures and Tables
Fig. 1
A 29-year-old man presented with fever and intractable headache.
(a) Brain CT depicts ill defined hypodensity lesion in the right basal ganglia and right internal capsule area with mild mass effect to ipsilateral ventricle.
(b and c) Brain MR T2 weighted coronal and axial images reveal confluent configuration of the abscess in right basal ganglia and internal capsule area extending to adjacent corona radiata and thalamus with perilesional edema upward to frontoparietal white matter, corpus callosum and downward to pons. Smaller satellite lesion was also noted at right parieto-occipital subcortical area.
(d) On Gadolinium-enhanced MR T1-weighted images, irregular wall enhancement along the margin of the abscess is noted.
(e) The center of the fungal abscess shows diffusion restriction presenting central high signal intensity within low signal intensity capsule on DWI.
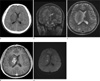
Fig. 2
Stereotactic Biopsy of the lesion with D-PAS staining on high power field shows acute inflammatory cells with hemorrhage and a few fungal hyphae. The fungal hyphae show true septations and branching pattern with acute angle, morphologically consistent with aspergillus.

Fig. 3
Follow up MRI was undertaken owing to deteriorated patient's status.
(a and b) Increased size of abscess in the right basal ganglia with aggravated perilesional edema on T2-weighted and Gondolinium-enhanced T1-weighted images is noted. Newly developed hydrocephalus in contralateral side is also demonstrated due to mass effect.
(c) Two weeks after modification in antifungal medication, additional brain CT depicted improvement of the lesional edema.
References
1. Beal MF, O'Carroll CP, Kleinman GM, Grossman RI. Aspergillosis of the nervous system. Neurology. 1982. 32:473–447.
2. Yuh WT, Nguyen HD, Gao F, Tali ET, Fisher DJ, Mayr NA, et al. Brain parenchymal infection in bone marrow transplantation patients: CT and MR findings. AJR Am J Roentgenol. 1994. 162:425–430.
3. Ashdown BC, Tien RD, Felsberg GJ. Aspergillosis of the brain and paranasal sinuses in immunocompromised patients: CT and MR imaging findings. AJR Am J Roentgenol. 1994. 162:155–159.
4. DeLone DR, Goldstein RA, Petermann G, Salamat MS, Miles JM, Knechtle SJ, et al. Disseminated aspergillosis involving the brain: distribution and imaging characteristics. AJNR Am J Neuroradiol. 1999. 20:1597–1604.
5. Kim DG, Hong SC, Kim HJ, Chi JG, Han MH, Choi KS, et al. Cerebral aspergillosis in immunologically competent patients. Surgical neurology. 1993. 40:326–331.
6. Narayan SK, Kumar K, Swaminathan RP, Roopeshkumar VR, Bhavna B. Isolated cerebral aspergilloma in a young immunocompetent patient. Practical neurology. 2009. 9:166–168.
7. Phuttharak W, Hesselink JR, Wixom C. MR features of cerebral aspergillosis in an immunocompetent patient: Correlation with histology and elemental analysis. AJNR Am J Neuroradiol. 2005. 26:835–838.
8. Siddiqui AA, Shah AA, Bashir SH. Craniocerebral aspergillosis of sinonasal origin in immunocompetent patients: clinical spectrum and outcome in 25 cases. Neurosurgery. 2004. 55:602–611. discussion 611-603.
9. Khachane SO, kumar V, Sanghvi DA. Central nervous system aspergillosis in immunocompetent patient. Eur J Radiol Extra. 2007. 63:53–56.
10. Yamada K, Zoarski GH, Rothman MI, Zagardo MT, Nishimura T, Sun CC. An intracranial aspergilloma with low signal on t2-weighted images corresponding to iron accumulation. Neuroradiology. 2001. 43:559–561.
11. Keyik B, Edguer T, Hekimoglu B. Conventional and diffusionweighted MR imaging of cerebral aspergillosis. Diagn Interv Radiol. 2005. 11:199–201.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download