Abstract
Purpose
To evaluate the geometry of carotid artery by assessing the images of contrast-enhanced MR angiography (CE-MRA) and interrelationships between the geometry of carotid artery and clinical factors.
Materials and Methods
216 consecutive patients who performed supraaortic CE-MRA with fast spoiled gradient-echo imaging were included. Their medical records were reviewed for variable information including risk factors predictive of generalized atherosclerotic disease (age, hypertension (HTN), diabetes mellitus, hyperlipidema, and smoking), sex, body weight, height, and body mass index (BMI). We reviewed the CE-MRA with carotid origin (3 types), carotid artery tortuosity, angle of internal carotid artery bifurcation, the type of aortic arch branching, and the presence of the coiling of carotid artery.
Results
Multinomial logistic regression analysis showed that significantly contributed clinical backgrounds for carotid origin were the age and the BMI. With an increase of age at 1, the probability that the type of carotid origin become from type 1 to type 2 was 0.9 times (p=0.004) in right carotid artery (RCA), 0.9 times (p=0.031) in left carotid artery (LCA), 0.9 times that are likely to be type3 from type 2 (p<0.001) in RCA and 0.9 times in LCA (p=0.009). Increase in BMI at 1 increased odds of becoming type 2 as 1.1 times (p=0.067) in RCA, 1.1 times (p=0.009) in LCA and increased chance of becoming type 3 as 1.2 times (p=0.001) in RCA, 1.2 times (p=0.003) in LCA. Mean value of right and left carotid tortuosity were 240.9±69.0° and 154.4±55.0°, respectively.
Figures and Tables
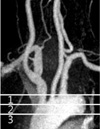 | Fig. 1Classification of the carotid origin
Type 1 carotid origin defined as the origin of innominate artery or left common carotid artery at upper 1/2 of the aortic arch; type 2 at their origin of lower 1/2 of the aortic arch; type 3 below the lower margin of the aortic arch
|
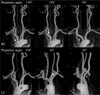 | Fig. 2Measurement of the carotid tortuosity
Method of carotid tortuosity (T) measurement: a+b+c = carotid tortuosity
|
References
1. Faggioli G, Ferri M, Gargiulo M, et al. Measurement and impact of proximal and distal tortuosity in carotid stenting procedures. J Vasc Surg. 2007. 46(6):1119–1124.
2. Kastrup A, Schulz JB, Raygrotzki S, Groschel K, Ernemann U. Comparison of angioplasty and stenting with cerebral protection versus endarterectomy for treatment of internal carotid artery stenosis in elderly patients. J Vasc Surg. 2004. 40(5):945–951.
3. Hobson RW, 2nd . Carotid artery stenting in octogenarians: the jury is still out. J Endovasc Ther. 2006. 13(3):310–311.
4. Lam RC, Lin SC, DeRubertis B, Hynecek R, Kent KC, Faries PL. The impact of increasing age on anatomic factors affecting carotid angioplasty and stenting. J Vasc Surg. 2007. 45(5):875–880.
5. Friedman MH, Deters OJ, Mark FF, Bargeron CB, Hutchins GM. Arterial geometry affects hemodynamics. A potential risk factor for athersoclerosis. Atherosclerosis. 1983. 46(2):225–231.
6. Corso L, Moruzzo D, Conte B, et al. Tortuosity, Kinking, and coiling of the carotid artery: expression of atherosclerosis or aging? Angiology. 1998. 49(5):361–371.
7. Madhwal S, Rajagopal V, Bhatt DL, Bajzer CT, Whitlow P, Kapadia SR. Predictors of difficult carotid stenting as determined by aortic arch angiography. J Invasive Cardiol. 2008. 20(5):200–204.
8. Lin SC, Trocciola SM, et al. Analysis of anatomic factors and age in patients undergoing carotid angioplasty and stenting. Ann Vasc Surg. 2005. 19(6):798–804.
9. Togay-Işikay C, Kim J, Betterman K, et al. Carotid artery tortousity, kinking, coiling: stroke risk factor, marker, or curiosity? Acta Neurol Belg. 2005. 105(2):68–72.
10. Pancera P, Ribul M, Presciuttini B, Lechi A. Prevalence of carotid artery kinking in 590 consecutive subjects evaluated by echocolordoppler. Is there a correlation with arterial hypertension? J Intern Med. 2000. 248(1):7–12.
11. Pancera P, Ribul M, De Marchi S, Arosio E, Lechi A. Prevalence of morphological alterations in cervical vessels: a colour duplex ultrasonographic study in a series of 3300 subjects. Int Angiol. 1998. 17(1):22–27.
12. Nederkoorn PJ, Elgersma OE, van der Graaf Y, Eikelboom BC, Kappelle LJ, Mali WP. Carotid artery stenosis: accuracy of contrast-enhanced MR angiography for diagnosis. Radiology. 2003. 228(3):677–682.
13. Randoux B, Marro B, Koskas F, Chiras J, Dormont D, Marsault C. Proximal great vessels of aortic arch: comparison of three-dimensional gadolinium-enhanced MR angiography and digital subtraction angiography. Radiology. 2003. 229(3):697–702.
14. Ho VB, Foo TK. Optimization of gadolinium-enhanced magnetic resonance angiography using an automated bolus-detection algorithm (MR SmartPrep). Original investigation. Invest Radiol. 1998. 33(9):515–523.
15. Faggioli GL, Ferri M, Freyrie A, et al. Aortic arch anomalies are associated with increased risk of neurological events in carotid stent procedures. Eur J Vasc Endovasc Surg. 2007. 33:436–441.
16. North American Symptomatic Carotid Endarterectomy Trial Collaborators. Beneficial effect of carotid endarterectomy in symptomatic patients with high-grade carotid stenosis. N Engl J Med. 1991. 325(7):445–453.
17. Wutke R, Fellner C, Fellner F, et al. 3D TOF turbo MRA of the carotid arteries: a fast alternative for imaging of atherosclerotic carotid disease. Eur Radiol. 2000. 10:126.
18. Fellner FA, Fellner C, Wutke R, et al. Fluoroscopically triggered contrast-enhanced 3D MR X-ray angiography and 3D time-of-flight turbo MRA of the carotid arteries: first clinical experiences in correlation with ultrasound, X-ray angiography, and endarterectomy findings. Magn Reson Imaging. 2000. 18(5):575–585.
19. Back MR, Rogers GA, Wilson JS, Johnson BL, Shames ML, Bandyk DF. Magnetic resonance angiography minimizes need for arteriography after inadequate carotid duplex ultrasound scanning. J Vasc Surg. 2003. 38(3):422–430.
20. Weibel J, Fields WS. Tortuosity, coiling, and kinking of the ICA. Etiology and radiographic anatomy. Neurology. 1965. 15:7–11.
21. Mochida M, Sakamoto H, Sawada Y, et al. Visceral fat obesity contributes to the tortuosity of the thoracic aorta on chest radiograph in poststroke Japanese patients. Angiology. 2006. 57(1):85–91.
22. Kornreich L, Hadar H, Sulkes J, et al. Effect of normal ageing on the sites of aortic bifurcation and inferior vena cava confluence: a CT study. Surg Radiol Anat. 1998. 20(1):63–68.
23. Leipzig TJ, Dohrmann GJ, et al. The tortous or kinked carotid artery: pathogenesis and clinical considerations. A historical review. Surg Neurol. 1986. 25(5):478–486.
24. Sugawara J, Hayashi K, Yokoi T, Tanaka H. Age-associated elongation of the ascending aorta in adults. JACC Cardiovasc Imaging. 2008. 1(6):739–748.
25. Wood NB, Zhao SZ, Zambanini A, et al. Curvature and tortuosity of the superficial femoral artery: a possible risk factor for peripheral arterial disease. J Appl Physiol. 2006. 101(5):1412–1418.
26. Hayashi K, Miyachi M, Seno N, et al. Variations in carotid arterial compliance during the menstrual cycle in young woman. Exp Physiol. 2006. 91(2):465–472.




 PDF
PDF ePub
ePub Citation
Citation Print
Print








 XML Download
XML Download