Abstract
Enteric duplications associated with the pancreas are especially uncommon, and the differential diagnosis of pancreatic duplication cysts is often difficult, and may be confused with various cystic lesions of the pancreas. We report a case of pancreatic duplication cyst; present the images and laboratory findings including cyst fluid tumor markers. MR and MRS findings enabled the detection of the location, contour, characteristics of cystic fluid and definition of tissue planes between the lesion and adjacent structures, providing useful information for an accurate surgical approach.
Enteric duplication is an ectopic cyst or a tubular structure composed of smooth muscles surrounding mucosa of the gastrointestinal tract (1). Most cases of duplication are present before the age of 2 years as an acute abdomen or bowel obstruction (2). Diagnosis of duplication cyst is uncommon in adults, and enteric duplication within the pancreas is especially uncommon and difficult to diagnose because it may be confused with pancreatic pseudocyst, mucinous cystic neoplasm, intraductal papillary mucinous neoplasm, choledochal cyst or lymphangioma. We report clinical findings and imaging of pancreatic duplication cyst in an adult. To our knowledge, this is the first reported case of a pancreatic duplication cyst in an adult demonstrated by MR imaging and MR Spectroscopy.
A 46-year-old woman with abdominal pain was referred to our hospital because of known pancreatic mass, diagnosed on ultrasonography 3 years ago. Her medical history was unremarkable. She previously had undergone tonsillectomy 5 years ago. On physical examination, the patient had a palpable abdominal mass on the right upper quadrant. Laboratory examinations revealed decreased serum hemoglobin (9.2µg/dl) and normal white cell count. The serum amylase, lipase, carbohydrate-associated antigen (CA) 19-9 and carcinoembryonic antigen (CEA) were within normal limits.
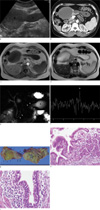
Ultrasonography and contrast enhanced computed tomography (CT) showed two homogeneous echogenic cystic masses, one 9 cm sized spherical shaped mass and the other one 11×3 cm sized tubular cystic mass each located in the pancreatic head and the body, respectively (Fig. 1a, b). MR imaging and MR spectroscopy (MRS) were performed (Sigma 1.5T GE. Milwaukee, WI, U.S.A.). T1-weighted (TR/TE, 100/4.2 msec) and T2-weighted (TR/TE, 1500/89 msec) MR images showed a well circumscribed cystic mass consisting of 9×7.3 cm sized spherical and 11×3.4 cm sized tubular cystic lesions in pancreatic head and body, respectively. The cystic lesions had homo-geneously hypointense fluid on T1-weighted images and hyperintense fluid within hypointense cystic wall on T2-weighted images (Fig. 1c, d). MRCP showed normal contour of the biliary duct and pancreatic duct without communication with the cystic lesions (Fig. 1e). In vivo MRS was performed with a free-breathing multivoxel point-resolved spectroscopy (PRESS) sequence (TR/TE=1500/30 msec, 2048-point acquisition, 2500 Hz bandwidth four averages). CHESS water suppression with 20 Hz bandwidth was obtained after semi-automated higher-order shimming. Outer volume suppression was achieved with six suppression bands placed three-dimensionaly around the lesions. Post-processing was applied automatically with zero-order phase correction, zero-filling to 4096 points, and noise was removed with a low pass 800-ms Gaussian filter. The voxel of interest (10×10×10 mm) was placed completely within the cyst. Spectroscopy revealed a short peak at 1.31 ppm, and there were no spectral peaks (Fig. 1f).
Laparoscopic subtotal pancreatectomy was performed. During the surgery, a smooth-walled cyst was identified in the pancreatic head and partially embedded in the pancreatic body. The whole specimen containing cystic mass was removed, and free distal portion of tubular cystic lesion was not communicated with the pancreatic mid-body and tail. Aspiration of the cystic contents yielded thick yellowish fluid. The fluid was transferred for analysis of CA19-9, and CEA levels. Marked elevated levels of CA19-9 (> 2400 U/ml) and CEA (> 880 ng/ml) were reported. The resected specimen was a dumbbell-shaped cystic mass filled with yellowish mucoid material partly covered with the pancreatic tissue and the inner surface of the cyst was partly covered with necrotic debris (Fig. 1g). Pathological examination was consistent with an enteric duplication, and it demonstrated mildly inflamed gastric or respiratory mucosa (Fig. 1h, i). The post-operative course was uneventful, and she was discharged on postoperative day 29.
Isolated duplication cyst is a rare congenital anomaly (1, 3), and occurs most commonly along the ileum, esophagus, or colon. Enteric duplication cyst contains smooth muscle layers and mucous membrane lining, occasionally gastric, intestinal, and respiratory epithelia. Enteric duplication typically lies on the paramesenteric border of the intestine, and generally do not communicate with the lumen, but may share smooth muscle layers with the adjacent bowel (1). However, it rarely occurs in such ectopic locations as the pancreas, biliary tree, and tongue. Although the embryology of duplication cyst remains unknown, enteric duplication cyst is thought to be the result of a diverticulum of the embryonic intestine that fails to undergo regression or defective recanalization of the alimentary tube during early fetal life (2). Both the stomach and pancreas arise as a bud from the primitive foregut. If the diverticulum forms on or near the pancreatic bud, a duplication cyst with pancreatic communication would result (4). The presenting symptoms are non-specific and can be revealed by complications such as infection, hematemesis, compression or carcinoma arising in the cyst (2). The symptoms of pancreatic duplication cyst are reported as recurrent abdominal pain caused either by recurrent pancreatitis or peptic ulceration (5).
When radiologic images show pancreatic cystic mass, the differential diagnosis includes all cystic masses such as pancreatic pseudocyst, cystadenoma, intraductal papillary mucinous neoplasm, choledochal cyst, cystic lymphangioma, etc. Ultrasonography usually demonstrates an anechoic mass with good through transmission due to clear fluid contents or can also produce complex internal echoes with hemorrhage and infection. CT or MRI show the full extent of the duplication cyst, excluding noncystic masses, presence of hemorrhage or protein, and additional anomalies (6). This case of duplication cyst was partially located in the pancreatic head and did not adhere to the pancreatic body and tail. Coexistence of spherical and tubular countered cysts are a highly suggestive findings of duplication cyst. Although the fluid within the duplication cyst has homogenous high signal intensity on T2-weighted MR images, variable patterns of signal intensity are seen on T1-weighted MR images because of the presence of protein or hemorrhage. ERCP is a useful tool for biliary and pancreatic ductal anatomy and demonstrates any communication between the duplication cyst and the main pancreatic duct to aid in planning surgical approach. MR cholangiography is a noninvasive way to detect biliary abnormalities and relationship between the duplication cyst, common bile duct and pancreatic duct (5).
On previous report, mediastinal foregut duplication cyst with in vivo 1H MRS on a 1.5T magnet showing a large metabolite peak at 2.02 ppm, attributable to N-acetylated compounds in addition to smaller peak at 1.33 ppm, was considered to represent lipids (7). In our case, a short peak was detected at 1.31 ppm and no other peaks were demonstrated. The mucus secreted by respiratory epithelium and the mucous glands of the foregut cyst contain-glycoproteins that have N-acetylhexosamines as components and lipid breakdown products that are thought to contribute to the observed spectrum (7).
The analysis of cystic fluid for the differential diagnosis of benign vs. premalignant or malignant pancreatic cystic lesions suggest that high CEA level predicts mucinous cystic neoplasm (2, 3, 8) and low levels of CEA or CA 19-9 suggest the presence of a serous cystadenoma or pseudocyst (8). In our case, the contents of the cyst revealed high levels of CEA and CA19-9, and there was no malignant lesion on pathologic examination. High concentrations of CEA and CA19-9 have been reported in a duplication cyst in the absence of malignancy (2). Although carcinoma arising from a duplication cyst is extremely rare, the production of oncofetal antigens by the epithelial lining of duplication cyst raises the problem of a precancerous condition in long standing intestinal duplications (2).
Duplication cyst must be completely excised because it often has been the cause of morbidity. Adenocarcinoma has been reported in foregut duplications (9). Complete local resection of enteric duplication cysts in the pancreatic head can be performed for definitive management, avoiding the complications of more radical procedures. Local resection can prevent the development of pancreatic insufficiency in the young patients. The optimal treatment procedure for an enteric duplication cyst varies considerably, depending on its location in the pancreas (10). Identifying the presence or absence of communication with the pancreatic duct is very important for selecting appropriate surgical procedure.
In conclusion, enteric duplication cyst of pancreas is a rare lesion in adult, and it should be included in the differential diagnosis of various cystic pancreatic lesions. We present a case of pancreatic duplication cyst, coexisting spherical and tubular cystic lesions partially located in the pancreatic head on radiologic images, that contains fluid with high CEA and CA 19-9 levels, and non-specific MR spectroscopy peaks.
Radiologists should be aware of this rare case of pancreatic cystic lesion and imaging findings for differential diagnosis, which is significantly helpful in terms of accurate preoperative diagnosis and obtaining information for planning proper surgical approach for this condition.
Figures and Tables
Fig. 1
(a) Ultrasound image shows a spherical (S) and tubular (T) cystic mass, containing relatively echogenic materials in spherical cyst of pancreatic head. (b) Contrast enhanced CT scan demonstrates a spherical and tubular cystic mass arising from the pancreatic head and partially invaded in body, and free distal portion of tubular cystic lesion (*). (c) Axial T1-weighted and (d) T2-weighted MR images reveal a hypointense and hyperintense cystic mass without septation or a mural nodule in the pancreatic head and body. (e) MRCP shows normal pancreatic main duct (arrowheads) without distension and a cystic mass (asterisk). (f) MR spectroscopy shows minimal peak in 1.31 ppm (*) and non-specific peaks. (g) Surgical specimen shows a dumbbell-shaped spherical (S, arrows) and tubular (T, arrowheads) mass partly covered by pancreatic tissue. It is filled with yellowish mucoid materials. Inner surface of the cyst is partly covered by necrotic debris. (h) Microscopic examination of the cystic mass shows mildly inflamed gastric antral and fundic mucosa along with intact submucosa, muscularis mucosa, and proper muscle coats (H&E ×200). (i) The other portion of cystic mass comprised of respiratory mucosa and underlying normal smooth muscle coats (H&E ×200).
References
1. Siddiqui AM, Shamberger RC, Filler RM, Perez-Atayde AR, Lillehei CW. Enteric duplications of the pancreatic head: definitive management by local resection. J Pediatr Surg. 1998. 33:1117–1120.
2. D'Journo XB, Moutardier V, Turrini O, et al. Gastric duplication in an adult mimicking mucinous cystadenoma of the pancreas. J Clin Pathol. 2004. 57:1215–1218.
3. Brugge WR, Lewandrowski K, Lee-Lewandrowski E, et al. Diagnosis of pancreatic cystic neoplasms: a report of the cooperative pancreatic cyst study. Gastroenterology. 2004. 126:1330–1336.
4. La Hei ER, Cohen RC. Antenatal detection of a pancreatic foregut duplication cyst. J Pediatr Surg. 2001. 36:501–502.
5. Wong AM, Wong HF, Cheung YC, Wan YL, Ng KK, Kong MS. Duodenal duplication cyst: MRI features and the role of MR cholangiopancreatography in diagnosis. Pediatr Radiol. 2002. 32:124–125.
6. Macpherson RI. Gastrointestinal tract duplications: clinical, pathologic, etiologic, and radiologic considerations. Radiographics. 1993. 13:1063–1080.
7. Santhosh K, Thomas B, Varma L, et al. Metabolite signature of developmental foregut cyst on in vivo and in vitro (1)H MR spectroscopy. J Magn Reson Imaging. 2008. 28:493–496.
8. van der Waaij LA, van Dullemen HM, Porte RJ. Cyst fluid analysis in the differential diagnosis of pancreatic cystic lesions: a pooled analysis. Gastrointestinal Endoscopy. 2005. 62:383–389.
9. Olsen JB, Clemmensen O, Andersen K. Adenocarcinoma arising in a foregut cyst of the mediastinum. Ann Thorac Surg. 1991. 51:497–499.
10. Kohno M, Ikawa H, Konuma K, Masuyama H, Fukumoto H, Ogawa E, et al. Laparoscopic enucleation of a gastroenteric duplication cyst arising in a pancreatic tail that did not communicate with the pancreatic duct: report of a case. Surg Today. 2010. 40:281–284.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download