Abstract
Various synthetic progestogens that are used as contraceptives have been reported to influence lipid and lipoprotein fractions differently. Depo-medroxyprogesterone acetate (DMPA), a synthetic progestogen, is used by Nepalese women as a contraceptive agent. Our study aims to determine the effects of long-term use of DMPA on lipid metabolism. We performed this study on 60 healthy Nepalese women who had been using DMPA for more than 2 yr and age- and weight-matched control subjects who were not using hormonal contraceptives. Fasting blood samples were collected from the subjects for the estimation of total cholesterol (TC) and triglyceride (TG) levels, and the levels of high-density lipoprotein cholesterol (HDL-C) and low-density lipoprotein cholesterol (LDL-C) were estimated using the Friedewald's equation. TC and LDL-C levels in DMPA users were significantly higher than those in non-users. Our study concluded that DMPA use induces lipid metabolism changes that can increase the risk of cardiovascular diseases.
Depo-medroxyprogesterone acetate (DMPA, Depo-Provera®) is a highly effective contraceptive with a very low failure rate [1]; however, women who use this contraceptive may experience some adverse effects. DMPA is a weak androgenic progestin that is administered as a single 150-mg intramuscular (i.m.) injection every 3 months. The contraceptive appears to be a potent inhibitor of gonadotrophins [2, 3].
There has been considerable debate about the effect of DMPA on lipid metabolism; studies have reported both increase and decrease in the levels of total cholesterol (TC), triglyceride (TG), and low-density lipoprotein cholesterol (LDL-C) in DMPA users. Many studies have also shown a decrease in high-density lipoprotein cholesterol (HDL-C) levels in long- and short-term DMPA users [4, 5]. Some studies have reported that DMPA does not exert any effect on various lipoproteins and lipid components [6, 7]. These DMPA-induced alterations in lipid metabolism can cause serious cardiovascular adverse effects in women. In this study, we aimed to identify the effects of DMPA use on the lipid profile of Nepalese women.
This study was performed at Tribhuvan University Teaching Hospital, Institute of Medicine (TUTH, IOM), Kathmandu, Nepal, from February to October 2008. We selected 60 women (25-35 yr of age) who weighed less than 75 kg, did not have any known diseases, and have not received any medication other than DMPA; these women were selected when they visited the family planning department of the hospital. They were regularly taking DMPA (150-mg (i.m.) injection) at 3-month intervals for more than 2 yr. We also recruited a control group of 100 age- and weight-matched healthy women (among the staff and students of TUTH, IOM) who were not using any hormonal contraceptives. Participants using lipid-lowering drugs were excluded from the study. After obtaining verbal consent from the subjects, fasting blood samples (5 mL) were collected from each subject to determine the plasma lipid and lipoprotein levels. This study was approved by our institutional review board. Human cholesterol liquicolor and triglyceride liquicolor mono kits were used for the enzymatic determination of TC [8] and TG [9] levels, respectively, in the Evolution 3000 (Biochemical Systems International, Arezzo, Italy) chemical analyzer. HDL-C was measured indirectly by using a precipitating reagent (Human HDL cholesterol; Wiesbaden, Germany) [10]. We strictly followed the reagent instruction manuals for all the assays. Concentrations of LDL-C were estimated using Friedewald's equation [11]. Both normal and high-level control sera were used in each run, and CV of imprecision was 4.1% for TC, 4.7% for TG, and 3.8% for HDL-C. Data were analyzed using SPSS software (SPSS 11.5 version Inc., Chicago, IL, USA). The means of the 2 groups were compared using the Student's t test, and the results were considered insignificant when P>0.05. All results are expressed as mean±SD.
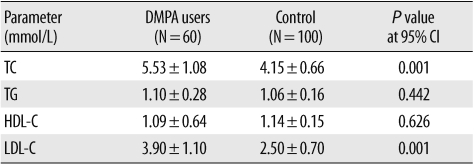
There were no significant differences in the age and weight of the control subjects and DMPA users. TC and LDL-C levels in DMPA users were significantly higher than those in the control group (Table 1). The HDL-C and TG levels were not significantly different from the previously reported levels.
The adverse effects of DMPA on lipid metabolism are related to its weak androgenic effect [12]. The studies on the effect of DMPA on plasma lipids have yielded inconsistent findings. In our study, the LDL-C and TC levels in DMPA users were significantly higher than those in the control subjects. This finding is in agreement with the previous studies [6, 13, 14]. Unlike our study, the studies by Grönroos and Lehtonen [3] and Fajumi [15] showed a decrease in TC levels, whereas those by Faddah et al. [6] and Garza-Flores et al. [5] did not show any changes in TC levels in DMPA users. Neither HDL-C nor TG levels were affected in DMPA users. However, many conflicting results have been reported [6, 12, 16]. Estrogens increase serum HDL-C levels and decrease serum TC and LDL-C levels, whereas progesterone has the opposite effect. DMPA, an weak synthetic progesterone, opposes the effect of estrogens. The level of HDL-C is inversely related to the incidence of cardiovascular diseases [17]. Although there was no significant decrease in the HDL-C levels of DMPA users in our study, there was an increase in the LDL-C levels. Our study concluded that DMPA induces lipid metabolism changes that can increase the risk of cardiovascular diseases.
References
1. Kupperman HS, Epstein JA. Medroxyprogesterone acetate in the treatment of constitutional sexual precocity. J Clin Endocrinol Metab. 1962; 22:456–458.

2. Laron Z, Rumney G, Rat L, Naji N. Effects of 17 alpha hydroxy-6alpha-methylprogesterone acetate (Depo-Provera) on urinary gonadotrophins and oestrogens in man. Acta Endocrinol (Copenh). 1963; 44:75–80. PMID: 14059893.
3. Grönroos M, Lehtonen A. Effect of high dose progestin on serum lipids. Atherosclerosis. 1983; 47:101–105. PMID: 6870984.

4. Fahmy K, Khairy M, Allam G, Gobran F, Alloush M. Effect of depo-medroxyprogesterone acetate on coagulation factors and serum lipids in Egyptian women. Contraception. 1991; 44:431–444. PMID: 1836755.
5. Garza-Flores J, De la Cruz DL, Valles de Bourges V, Sanchez-Nuncio R, Martinez M, Fuziwara JL, et al. Long-term effects of depot-medroxyprogesterone acetate on lipoprotein metabolism. Contraception. 1991; 44:61–71. PMID: 1832626.

6. Faddah LM, Al-Rehany MA, Abdel-Hamid NM, Bakeet AA. Oxidative stress, lipid profile and liver functions in average Egyptian long term depo medroxy progesterone acetate (DMPA) users. Molecules. 2005; 10:1145–1152. PMID: 18007380.

7. Kaunitz AM. Long-acting injectable contraception with depot medroxyprogesterone acetate. Am J Obstet Gynecol. 1994; 170:1543–1549. PMID: 8178904.

8. Deeg R, Ziegenhorn J. Kinetic enzymic method for automated determination of total cholesterol in serum. Clin Chem. 1983; 29:1798–1802. PMID: 6577981.

9. Bucolo G, David H. Quantitative determination of serum triglycerides by the use of enzymes. Clin Chem. 1973; 19:476–482. PMID: 4703655.

10. Assmann G, Schriewer H, Schmitz G, Hägele EO. Quantification of high-density-lipoprotein cholesterol by precipitation with phosphotungstic acid/MgCl2. Clin Chem. 1983; 29:2026–2030. PMID: 6640896.

11. Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972; 18:499–502. PMID: 4337382.

12. Godsland IF, Crook D, Simpson R, Proudler T, Felton C, Lees B, et al. The effects of different formulations of oral contraceptive agents on lipid and carbohydrate metabolism. N Engl J Med. 1990; 323:1375–1381. PMID: 2146499.

13. Kongsayreepong R, Chutivongse S, George P, Joyce S, McCone JM, Garza-Flores J, et al. World Health Organization. Task Force on Long-Acting Systemic Agents for Fertility Regulation Special Programme of Research, Development and Research Training in Human Reproduction. A multicentre comparative study of serum lipids and apolipoproteins in long-term users of DMPA and a control group of IUD users. Contraception. 1993; 47:177–191. PMID: 8449018.

14. Mostafavi H, Abdali K, Zare N, Rezaian GR, Ziyadlou S, Parsanejad ME. A comparative analysis of three methods of contraception: Effects on blood glucose and serum lipid profiles. Ann Saudi Med. 1999; 19:8–11. PMID: 17337976.

15. Fajumi JO. Alterations in blood lipids and side effects induced by depo-provera in Nigerian women. Contraception. 1983; 27:161–175. PMID: 6221882.

16. Enk L, Landgren BM, Lindberg UB, Silfverstolpe G, Crona N. A prospective, one-year study on the effects of two long acting injectable contraceptives (depot-medroxyprogesterone acetate and norethisterone oenanthate) on serum and lipoprotein lipids. Horm Metab Res. 1992; 24:85–89. PMID: 1533383.

17. Miller NE, Thelle DS, Forde OH, Mjos OD. The Tromso heart-study. High-density lipoprotein and coronary heart-disease: a prospective case-control study. Lancet. 1977; 1:965–968. PMID: 67464.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download