Abstract
Background
Clopidogrel has been widely used to prevent recurrent ischemia in patients with acute coronary syndrome (ACS). However, inter-individual variability in response to clopidogrel has been a problem in the clinical setting. The aim of the present study was to investigate the frequency of clopidogrel resistance and to determine the clinical, pharmacokinetic, and pharmacogenetic factors for clopidogrel resistance in Korean patients with ACS.
Methods
Clinical information, such as the underlying diseases and concurrent medications, of 114 patients with ACS who received clopidogrel therapy was studied. The degree of inhibition of platelets was assessed using the VerifyNow assay (Accumetrics, USA). The patients who showed less than 20% inhibition of platelets were defined as non-responders to clopidogrel treatment. Steady state plasma concentrations of clopidogrel were measured using HPLC/tandem mass spectrometry. CYP2C19 genotyping was also performed.
Results
A wide inter-individual variability was observed in platelet inhibition (0-76%); 56 patients (49%) showed less than 20% inhibition. There were no differences between the patients' history of diabetes mellitus and concurrent medications as well as the plasma concentrations of clopidogrel of the responders and non-responders. CYP2C19 variants, including CYP2C19*2 and CYP2C19*3, were more commonly observed in the non-responders than in the responders (P value<0.0001).
Conclusions
The response to clopidogrel was highly variable in Korean patients with ACS. The results of the present study confirmed that the genetic polymorphism of CYP2C19 could be important in clopidogrel response. However, further studies are required to investigate other likely factors involved in clopidogrel resistance.
Platelet activation and aggregation are the underlying pathophysiological mechanisms associated with acute coronary syndrome (ACS) and other ischemic conditions. Clopidogrel has been recommended as an antiplatelet agent for the prevention of recurrent ischemic events. It is a prodrug that is converted to an active metabolite by the hepatic cytochrome P450 (CYP)-dependent pathway [1, 2]. The active thiol metabolite irreversibly binds to the P2Y12 ADP receptor of the platelets, which results in the inhibition of platelet aggregation [1, 2].
Many clinical studies have shown that the antiplatelet response to clopidogrel treatment has wide inter-individual variability, which may be associated with the poor clinical outcome in some patients [3-6]. Recent studies on the me-chanisms associated with clopidogrel resistance have shown that genetic polymorphisms, noncompliance, inappropriate dosage, obesity, insulin resistance, and drug-drug interactions might play an important role in the development of clopidogrel resistance [1, 2, 5, 7-9]. CYP variants involved in the metabolic activation of clopidogrel, such as CYP3A4, CYP3A5, and CYP2C19, might also play an important role in clopidogrel resistance [1, 2, 5, 8]. The CYP2C19*2 and CYP2C19*3 variants have been reported to be significantly associated with clopidogrel resistance [1, 2, 4, 8]. The aim of this study was to investigate the frequency of clopidogrel resistance in Korean patients with ACS and to evaluate the relationship between the antiplatelet effects of clopidogrel and pharmacogenetic factors. The effects of clinical and pharmacokinetic factors on clopidogrel resistance were also investigated.
A total of 114 patients diagnosed with ACS at the Samsung Medical Center between June 2008 and July 2009 were evaluated. All the patients underwent coronary angiography and received a daily dose of 75 mg (105 patients) or 150 mg (9 patients) of clopidogrel for more than a month. The ages of the patients ranged from 35 to 87 yr (median age, 63 yr) and their body weights ranged from 43 to 105 kg (median weight, 69 kg). All the patients were undergoing dual antiplatelet therapy with aspirin and clopidogrel. Of the 114 patients, 32 received an additional dose of cilostazol. This study was approved by the Institutional Review Board of Samsung Medical Center, Seoul, Korea. Written informed consent was provided by all the patients.
All blood samples were collected at a steady state before the next administration of clopidogrel. The plasma concentrations of clopidogrel were determined using HPLC (HPLC 1100 system; Agilent, Santa Clara, CA , USA) coupled with tandem mass spectrometry (MS/MS) (API 4000; Applied Biosystems, Foster City, CA, USA). Chromatographic separation was performed on a C18 column (2.1×50 mm, 3 µm, Atlantis dC18, Waters; Milford, MA, USA). The mobile phase consisted of deionized water and acetonitrile with 0.1% formic acid, and the flow rate was 0.25 mL/min. The mass spectrometry analysis was performed in the multiple reaction monitoring mode, where the precursor-to-product ion transition was monitored at mass-to-charge ratio (m/z) 322.1→212.1 for clopidogrel and m/z 326.1→216.1 for the internal standard (2H4-clopidogrel). The CV values for intra- and inter-day precision were less than 10%, and the calibration curve ranged from 1 to 1,000 pg/mL.
The platelet function test was performed to monitor the antiplatelet effects of clopidogrel. The degree of platelet inhibition was determined by the VerifyNow P2Y12 assay (Accumetrics, San Diego, CA, USA). Data on ADP-induced platelet aggregation and clopidogrel-induced platelet inhibition were expressed in terms of platelet reactivity units and percent inhibition, respectively.
Genomic DNA was extracted from whole-blood leukocytes by using the Wizard Genomic DNA Purification Kit (Promega, Madison, WI, USA). Exon 4 and exon 5 of the CYP2C19 gene were isolated; CYP2C19*2 (681G>A; rs4244285) and CYP2C19*3 (636G>A; rs4986893) were amplified using PCR and a thermal cycler (Model 9700; Applied Biosystems). After treatment with shrimp alkaline phosphatase and exonuclease I, direct sequencing was performed using an ABI Prism 3100 Genetic Analyzer (Applied Biosystems) with a BigDye Terminator Cycle Sequencing Ready Reaction Kit (Applied Biosystems).
All statistical analyses were performed using MedCalc (MedCalc software; Mariakerke, Belgium). The findings for the non-responder and responder groups were compared using the Chi-square and Mann-Whitney tests. P values less than 0.05 were considered statistically significant.
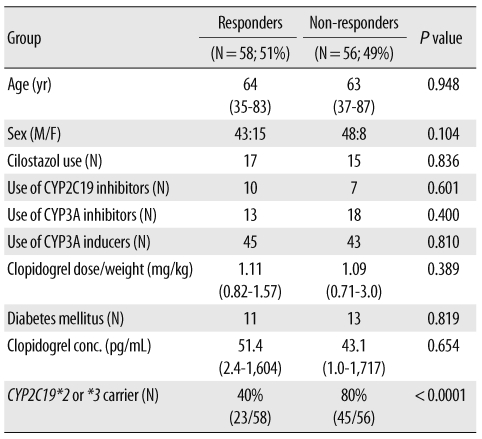
Among the 114 patients evaluated in this study, 68 (60%) were carriers for the CYP2C19*2 or CYP2C19*3 (Table 1).
There was a wide inter-individual variability in platelet inhibition (0-76%), and 56 patients (49%) showed less than 20% inhibition. Non-responders to clopidogrel treatment were more frequently found among patients with the variant CYP2C19 genes (P value<0.0001) (Table 1 and Fig. 1).
Clopidogrel concentrations ranged from 1 to 1,717 pg/mL (median, 39.5 pg/mL). There was no correlation between clopidogrel concentration and the weight-adjusted dose (data not shown). There was no difference between the clopidogrel concentrations in the responder and non-responder groups. Regarding the concentration of clopidogrel on the basis of the CYP2C19 genotype, the median concentrations of clopidogrel were 92.3 pg/mL and 34.1 pg/mL in patients with the wild-type and CYP2C19 variants, respectively (P value=0.0574).
In addition, other factors such as diabetes mellitus (DM) and concurrent medications, such as CYP2C19 inhibitors, CYP3A inhibitors, or CYP3A inducers, did not differ between the 2 study groups.
The goal of the present study was to evaluate the frequency of clopidogrel resistance and to comprehensively investigate the clinical, pharmacokinetic, and pharmacogenetic factors associated with clopidogrel resistance in Korean patients with ACS. Clopidogrel resistance was observed in 53 patients (46%) with ACS in this study. The frequency of clopidogrel resistance has been reported to range from 5% to 44% [8, 10, 11]. Clopidogrel resistance was believed to be caused by various clinical, pharmacokinetic, and pharmacogenetic factors [1, 2, 5, 7-9]. In this study, CYP2C19 genotypes were observed to have a significant association with clopidogrel resistance in Korean patients with coronary artery disease (CAD).
CYP2C19*2 is a mutation caused by the substitution of guanine with adenine at nucleotide position 681 of exon 5, which results in aberrant splicing and complete loss of CY-P2C19 activity [2, 9]. CYP2C19*3 is a mutation caused by a guanine to adenine point mutation at nucleotide position 636 of exon 4, which produces a premature stop codon [12]. Previous studies have suggested a correlation between CYP2C19*2 and clopidogrel resistance; however, most of these studies have been performed in patients from the Caucasian region, and there is very limited data on Asian populations. A previous study reported that only the CYP2C19*3 genotype was a significant risk factor for clopidogrel resistance among Korean patients with the CYP1A1, CYP2A2, CYP3A4, CYP3A5, CYP2C19, CYP2J2, and P2RY12 genotypes [8].
To investigate the pharmacokinetic factors associated with clopidogrel resistance, we quantified the plasma concentration of the prodrug clopidogrel. In procedures for quantification of active metabolites, a stabilization protocol for the unstable compounds is required [13, 14]. Although the use of special techniques for stabilizing the active metabolites was not possible in a routine clinical setting, high concentrations of the prodrug clopidogrel most likely indicated low rates of conversion of the prodrug to the active metabolite; thus, the antiplatelet effects were expected to be minimal in patients with high concentrations of clopidogrel. Patients who received clopidogrel therapy showed a wide distribution of clopidogrel concentrations. However, there was no correlation between the concentration and the weight-adjusted dose. Clopidogrel concentrations showed no correlation with other factors such as percent inhibition of platelets and CYP2C19 variants. The possible reasons for the lack of difference in clopidogrel concentrations on the basis of the CYP2C19 genotype are as follows: the potential effects of genetic polymorphisms other than CYP2C19; various clinical factors such as compliance, obesity, DM, and underlying characteristics of CAD; measurement of the prodrug clopidogrel concentration, which acted an indirect surrogate marker, rather than the active metabolite concentration; the factors associated with the sampling time for drug monitoring; and the small number of study subjects.
Pharmacodynamic response to clopidogrel was diminished by the genetic variations, but an association between the CYP2C19 genotype and clopidogrel concentrations was not observed in this study. Only 15% of clopidogrel is converted to active thiol metabolites by the CYP enzymes, while the remaining 85% is converted to inactive metabolites by esterases [5]. In addition, the levels of the prodrug clopidogrel were extremely low in comparison with the levels of the active and inactive metabolites. Other genes associated with absorption (ABCB1) and activation (CYP3A5) could also influence clopidogrel concentration. The possibility of the metabolic pathways influencing the clopidogrel response cannot be excluded. We also evaluated the possibility of drug-drug interactions leading to differences in the pharmacokinetic and pharmacodynamic characteristics of clopidogrel. Concurrent medications, such as CYP3A inducers or inhibitors, were not associated with the clopidogrel concentration or clopidogrel resistance. The clinical factors associated with clopidogrel resistance include noncompliance and insulin resistance [1]. Patients with DM have been reported to have significantly lower levels of active metabolites [1]. However, the 24 patients with DM who were on medication did not show differences in clopidogrel response in this study.
The study had the following limitations: First, only 2 allelic variants of CYP2C19 were studied. Other genes in the CYP group might also be involved in the metabolism of clopidogrel [1, 2, 7, 9]. Second, the plasma concentrations of the active thiol metabolites were not measured. Third, the number of patients was small.
In conclusion, this is the first study to evaluate the clinical, pharmacokinetic, and pharmacogenetic characteristics of clopidogrel resistance in Korean patients with ACS; the evaluations included the monitoring of clopidogrel concentrations. The results of this study confirmed that the genetic polymorphism of CYP2C19 could be important in clopidogrel response. However, further studies are required to investigate other likely factors involved in clopidogrel resistance.
Acknowledgement
This study was supported by a grant from the Korean Ministry of Education, Science, and Technology (FPR08A2-130 of the 21C Frontier Functional Proteomics Program) and a grant from the Korea Healthcare Technology R&D Project, Ministry of Health, Welfare & Family Affairs, Republic of Korea (A030001) and a grant from IN-SUNG Foundation for Medical Research (CB08071).
References
1. Gurbel PA, Antonino MJ, Tantry US. Recent developments in clopidogrel pharmacology and their relation to clinical outcomes. Expert Opin Drug Metab Toxicol. 2009; 5:989–1004. PMID: 19575629.

2. Gurbel PA, Tantry US, Shuldiner AR, Kereiakes DJ. Genotyping: one piece of the puzzle to personalize antiplatelet therapy. J Am Coll Cardiol. 2010; 56:112–116. PMID: 20471192.
3. Bonello L, Camoin-Jau L, Arques S, Boyer C, Panagides D, Wittenberg O, et al. Adjusted clopidogrel loading doses according to vasodilator-stimulated phosphoprotein phosphorylation index decrease rate of major adverse cardiovascular events in patients with clopidogrel resistance: a multicenter randomized prospective study. J Am Coll Cardiol. 2008; 51:1404–1411. PMID: 18387444.
4. Gurbel PA, Becker RC, Mann KG, Steinhubl SR, Michelson AD. Platelet function monitoring in patients with coronary artery disease. J Am Coll Cardiol. 2007; 50:1822–1834. PMID: 17980247.

5. Simon T, Verstuyft C, Mary-Krause M, Quteineh L, Drouet E, Méneveau N, et al. Genetic determinants of response to clopidogrel and cardiovascular events. N Engl J Med. 2009; 360:363–375. PMID: 19106083.

6. Angiolillo DJ, Alfonso F. Platelet function testing and cardiovascular outcomes: steps forward in identifying the best predictive measure. Thromb Haemost. 2007; 98:707–709. PMID: 17938790.

7. Sibbing D, Gebhard D, Koch W, Braun S, Stegherr J, Morath T, et al. Isolated and interactive impact of common CYP2C19 genetic variants on the antiplatelet effect of chronic clopidogrel therapy. J Thromb Haemost. 2010; 8:1685–1693. PMID: 20492469.
8. Lee JM, Park S, Shin DJ, Choi D, Shim CY, Ko YG, et al. Relation of genetic polymorphisms in the cytochrome P450 gene with clopidogrel resistance after drug-eluting stent implantation in Koreans. Am J Cardiol. 2009; 104:46–51. PMID: 19576320.

9. Collet JP, Hulot JS, Pena A, Villard E, Esteve JB, Silvain J, et al. Cytochrome P450 2C19 polymorphism in young patients treated with clopidogrel after myocardial infarction: a cohort study. Lancet. 2009; 373:309–317. PMID: 19108880.

10. Mobley JE, Bresee SJ, Wortham DC, Craft RM, Snider CC, Carroll RC. Frequency of nonresponse antiplatelet activity of clopidogrel during pretreatment for cardiac catheterization. Am J Cardiol. 2004; 93:456–458. PMID: 14969622.

11. Angiolillo DJ, Fernandez-Ortiz A, Bernardo E, Ramírez C, Barrera-Ramirez C, Sabaté M, et al. Identification of low responders to a 300-mg clopidogrel loading dose in patients undergoing coronary stenting. Thromb Res. 2005; 115:101–108. PMID: 15567460.

12. De Morais SM, Wilkinson GR, Blaisdell J, Meyer UA, Nakamura K, Goldstein JA. Identification of a new genetic defect responsible for the polymorphism of (S)-mephenytoin metabolism in Japanese. Mol Pharmacol. 1994; 46:594–598. PMID: 7969038.
13. Bouman HJ, Parlak E, van Werkum JW, Breet NJ, ten Cate H, Hackeng CM, et al. Which platelet function test is suitable to monitor clopidogrel responsiveness? A pharmacokinetic analysis on the active metabolite of clopidogrel. J Thromb Haemost. 2010; 8:482–488. PMID: 20040042.

14. Takahashi M, Pang H, Kawabata K, Farid NA, Kurihara A. Quantitative determination of clopidogrel active metabolite in human plasma by LC-MS/MS. J Pharm Biomed Anal. 2008; 48:1219–1224. PMID: 18829199.





 PDF
PDF ePub
ePub Citation
Citation Print
Print



 XML Download
XML Download