Abstract
We present a rare case of microgranular variant acute promyelocytic leukemia (APL) associated with ider(17)(q10)t(15;17)(q22;q12) of an old-age patient. The initial chromosome study showed a 46,XX,del(6)(?q21q25),der(15)t(15;17)(q22;q12),ider(17)(q10)t(15;17)[10]/47,sl,+ider(17)(q10)t(15;17)[3]/46,XX[16]. FISH signals from a dual color dual fusion translocation PML-RARA probe were consistent with the results of conventional cytogenetics. Because of the rarity of ider(17)(q10)t(15;17) in microgranular APL, further studies on both gene dosage effect of this chromosomal abnormality and the influence of ider(17)(q10)t(15;17) on clinical features such as prognosis, survival, and treatment response of APL cases are recommended.
Acute promyelocytic leukemia (APL) is one of the most characteristic subtypes of AML in which abnormal promyelocytes predominate within peripheral blood or bone marrow [1]. Also, t(15;17)(q22;q21) shows a characteristic chromosomal translocation in APL, observable in 70-90% of APL patients. Owing to all trans-retinoic acid (ATRA) combined with chemotherapy, APL has one of the highest cure rates of all types of AML. Seventy to eighty percent of newly diagnosed APL patients with the PML-RARA rearrangement are cured or under long-term remission, yet some of them have a poor prognosis [2-5]. Because cytogenetics is one of the most powerful prognostic factors for the outcome of AML, cytogenetic abnormalities can cause change in treatment response, relapse, and clinicopathological characteristics [6]. Incidence of secondary cytogenetic abnormalities has been observed in ~40% of APL cases [1], but their prognostic significance is still unclear [5-7].
About 1% of the reported secondary cytogenetic abnormalities in APL patients are ider(17)(q10)t(15;17)(q22;q12), an infrequent type of additional recurrent chromosomal abnormality, according to a recent study [6]. However, ider(17)(q10)t(15;17) associated with the PML-RARA rearrangement in microgranular variant APL is even more rare. As far as we know, only 2 cases of the ider(17)(q10)t(15;17) abnormality in microgranular APL have been previously reported [8, 9]. Here, we describe an unusual microgranular APL case associated with ider(17)(q10)t(15;17), identified by both conventional cytogenetics and FISH analyses at the initial diagnosis.
A 59-yr-old woman who had previously been diagnosed with cerebral infarction was brought to our hospital due to right side weakness in November 2007. The initial complete blood count showed pancytopenia, Hb level of 9.9 g/dL (reference range 12-16 g/dL), platelet count of 83,000/µL (reference range 150,000-350,000/µL), and white blood cell count of 1,000/µL (reference range 4,000-10,000/µL). Bone marrow aspiration showed a hypercellular marrow replaced by increased promyelocytes with a paucity or absence of granules, accounting for 36% of all nucleated cells (Fig. 1). The results of special staining of bone marrow specimens were as follows: Myeloperoxidase, positive; periodic acid Schiff, negative; Nonspecific esterase, negative. Flow cytometric analysis was conducted and showed that the blasts were positive for CD13 (91.1%), CD33 (83.9%), CD117 (59.2%), CD2 (43.9%), and CD45 (25.4%), and negative for HLA-DR (3.4%), CD3 (1.3%), CD7 (0.6%), CD10 (1.8%), CD14 (2.4%), CD19 (5.1%), CD34 (1.4%), CD41 (2.9%), CD56 (1.2%), and TdT (0.9%). Bone marrow chromosome analysis revealed a 46,XX,del(6)(?q21q25),der(15)t(15;17)(q22;q12),ider(17)(q10)t(15;17)[10]/47,sl,+ider(17)(q10)t(15;17)[3]/46,XX[16] (Fig. 2). FISH signals from PML-RARA probes (Abbott Molecular/Vysis, Des Plaines, IL, USA) yielded the results of nuc ish(PML, RARA)×4(RARA con PML×3)[24/138], (PML, RARA)×6(RARA con PML×5)[14/138], (PML, RARA)×3(RARA con PML×2)[13/138], consistent with the abnormal fusion signal patterns seen in 37% of the nuclei examined (Fig. 2). The patient was diagnosed with APL and treated with induction chemotherapy consisting of daunorubicin, cytosine arabinoside, and ATRA. After completing induction chemotherapy, follow up bone marrow examination in January 2008 showed no evidence of morphologically visible residual leukemia. The concurrent karyotype analysis result was 46,XX in all analyzed cells; and PML-RARA FISH showed "nuc ish (PML, RARA)×2 [248]" in which the abnormal signal pattern was not observed. There was no evidence of a PML-RARA fusion gene in the reverse transcriptase-PCR (RT-PCR) analysis. As indicated by follow-up bone marrow biopsies conducted until September 2008, the patient remained in complete remission. During this period, the RT-PCR analysis did not show any signs of the PML-RARA fusion gene while other cytogenetic studies also indicated normal findings.
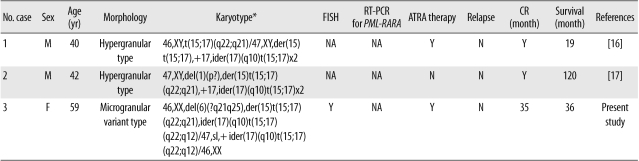
APL is a distinct subtype of AML and constitutes about 5-8% of all cases of AML diagnosis. According to the 2008 WHO classification, APL can be diagnosed when there is a t(15;17) or a PML-RARA rearrangement, even if peripheral blood or bone marrow studies show less than 20% promyelocytes [1]. As recently reported by Manola et al. [10] and our study group, the ider(17)(q10)t(15;17), an isochromosomal abnormality that occurs on the long arm of ider(17)t(15;17) after reciprocal translocation of t(15;17), is a relatively rare type of an additional recurrent cytogenetic abnormality that has been reported in 62 APL patients worldwide [8-13]. According to these studies, the influence of ider(17)(q10)t(15; 17) on the prognosis of adult APL patients is less significant than its effect on children. Indeed, 4 previously reported APL cases in children were all related to poor prognosis [8, 13-15], inferring that a more close and careful interpretation is necessary for childhood APL cases [13]. What is interesting is that so far, reports of ider(17)(q10)t(15;17) from microgranular variant (AML-M3v) type are extremely rare. Out of 62 total cases, information on APL morphology type were available in 42 cases, and most of these cases (40/42) were of the hypergranular APL type, except for 2 cases that clearly indicated AML-M3v (Table 1) [8, 9]. Therefore, further research is required to determine whether ider(17)(q10) and AML-M3v have a low association, and more careful observation should be conducted to prevent underestimating AML-M3v patients among ider(17)(q10)t(15;17) cases. Furthermore, double ider(17) (q10)t(15;17) is so rare in the International Public Databases that only 2 cases of APL patients indicating double ider(17)(q10)t(15;17) chromosomal abnormalities have been reported (Table 2) [16, 17]. In double ider(17)(q10)t(15;17), a gene dosage effect is observed owing to chromosomal abnormalities such as the PML-RARA fusion gene on chromosome 17 or the quadruplication of der(17q). In addition, since the deletion of the tumor suppressor gene TP53 occurs by the loss of 17p, further research is necessary to resolve the adverse prognosis of the APL group related to such copy number variations. Owing to the limited amount of clinical data in the literature, the relatedness between double ider(17)(q10)t(15;17) and an adverse prognosis is still unclear [16, 17]. In the case of our patient, it was hard to determine a strong association between the additional genetic aberration and prognosis because of the small clonal size of the "double ider(17)(q10)t(15;17)" abnormality.
Nevertheless, at least from a diagnostic perspective and as indicated in the authors' recent studies [13, 18], minimal residual disease detection using such multiple abnormal fusion signals through the PML-RARA FISH analysis in APL patients associated with ider(17)(q10)t(15;17) or double ider(17)(q10)t(15;17) would be considered to be a useful follow-up marker in clinical laboratories or hospitals. Additional study would contribute toward a better understanding of the influence of ider(17)(q10)t(15;17) on the prognosis, survival, and treatment response of such APL cases in adults or children. To the best of our knowledge, however, this is the third case report of microgranular variant APL associated with ider(17)(q10)t(15;17).
Acknowledgement
This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science, and Technology (2010-0023093).
References
1. Swerdlow SH, Campo E, editors. WHO classification of tumours of haematopoietic and lymphoid tissues. 2008. 4th ed. Lyon: IARC;p. 112–114.
2. Lengfelder E, Saussele S, Weisser A, Büchner T, Hehlmann R. Treatment concepts of acute promyelocytic leukemia. Crit Rev Oncol Hematol. 2005; 56:261–274. PMID: 16236522.

3. Sirulnik LA, Stone RM. Acute promyelocytic leukemia: current strategies for the treatment of newly diagnosed disease. Clin Adv Hematol Oncol. 2005; 3:391–397. 429PMID: 16167012.
4. Xu L, Zhao WL, Xiong SM, Su XY, Zhao M, Wang C, et al. Molecular cytogenetic characterization and clinical relevance of additional, complex and/or variant chromosome abnormalities in acute promyelocytic leukemia. Leukemia. 2001; 15:1359–1368. PMID: 11516096.

5. Hiorns LR, Swansbury GJ, Mehta J, Min T, Dainton MG, Treleaven J, et al. Additional chromosome abnormalities confer worse prognosis in acute promyelocytic leukaemia. Br J Haematol. 1997; 96:314–321. PMID: 9029019.

6. Cervera J, Montesinos P, Hernández-Rivas JM, Calasanz MJ, Aventín A, Ferro MT, et al. Additional chromosome abnormalities in patients with acute promyelocytic leukemia treated with all-trans retinoic acid and chemotherapy. Haematologica. 2010; 95:424–431. PMID: 19903674.

7. Hernández JM, Martín G, Gutiérrez NC, Cervera J, Ferro MT, Calasanz MJ, et al. Additional cytogenetic changes do not influence the outcome of patients with newly diagnosed acute promyelocytic leukemia treated with an ATRA plus anthracyclin based protocol. A report of the Spanish group PETHEMA. Haematologica. 2001; 86:807–813. PMID: 11522536.
8. Chou WC, Tang JL, Yao M, Liang YJ, Lee FY, Lin MT, et al. Clinical and biological characteristics of acute promyelocytic leukemia in Taiwan: a high relapse rate in patients with high initial and peak white blood cell counts during all-trans retinoic acid treatment. Leukemia. 1997; 11:921–928. PMID: 9204969.

9. Kaleem Z, Watson MS, Zutter MM, Blinder MA, Hess JL. Acute promyelocytic leukemia with additional chromosomal abnormalities and absence of Auer rods. Am J Clin Pathol. 1999; 112:113–118. PMID: 10396293.

10. Manola KN, Karakosta M, Sambani C, Terzoudi G, Pagoni M, Gatsa E, et al. Isochromosome der(17)(q10)t(15;17) in acute promyelocytic leukemia resulting in an additional copy of the RARA-PML fusion gene: report of 4 cases and review of the literature. Acta Haematol. 2010; 123:162–170. PMID: 20224268.
11. Kim M, Lee SA, Park HI, Oh EJ, Park CW, Lim J, et al. Two distinct clonal populations in acute promyelocytic leukemia, one involving chromosome 17 and the other involving an isochromosome 17. Cancer Genet Cytogenet. 2010; 197:185–188. PMID: 20193853.

12. Sainty D, Liso V, Cantù-Rajnoldi A, Head D, Mozziconacci MJ, Arnoulet C, et al. A new morphologic classification system for acute promyelocytic leukemia distinguishes cases with underlying PLZF/RARA gene rearrangements. Group Français de Cytogénétique Hématologique, UK Cancer Cytogenetics Group and BIOMED 1 European Coomunity-Concerted Acion "Molecular Cytogenetic Diagnosis in Haematological Malignancies.". Blood. 2000; 96:1287–1296. PMID: 10942370.
13. Kim MJ, Yoon HS, Cho SY, Lee HJ, Suh JT, Lee J, et al. ider(17)(q10)t(15;17) associated with relapse and poor prognosis in a pediatric patient with acute promyelocytic leukemia. Cancer Genet Cytogenet. 2010; 201:116–121. PMID: 20682396.

14. Simmers RN, Webber LM, Shannon MF, Garson OM, Wong G, Vadas MA, et al. Localization of the G-CSF gene on chromosome 17 proximal to the breakpoint in the t(15;17) in acute promyelocytic leukemia. Blood. 1987; 70:330–332. PMID: 2439153.

15. Prigogina EL, Fleischman EW, Puchkova GP, Mayakova SA, Volkova MA, Protasova AK, et al. Chromosomes in acute nonlymphocytic leukemia. Hum Genet. 1986; 73:137–146. PMID: 3721500.

16. Schoch C, Haase D, Haferlach T, Freund M, Link H, Lengfelder E, et al. Incidence and implication of additional chromosome aberrations in acute promyelocytic leukaemia with translocation t(15;17)(q22;q21): a report on 50 patients. Br J Haematol. 1996; 94:493–500. PMID: 8790148.

17. Qiu HR, Li JY, Miao KR, Wang R, Xu W. Clinical and laboratory studies of an acute promyelocytic leukemia patient with double ider(17q) chromosome aberration. Cancer Genet Cytogenet. 2008; 184:74–75. PMID: 18558295.

18. Kim MJ, Yoon HS, Lim G, Kim SY, Lee HJ, Suh JT, et al. ABL1 gene deletion without BCR/ABL1 rearrangement in a young adolescent with precursor B-cell acute lymphoblastic leukemia: clinical study and literature review. Cancer Genet Cytogenet. 2010; 196:184–188. PMID: 20082857.

Fig. 1
Bone marrow aspiration showing abnormal promyelocytes with sparse and/or fine granulation (dotted arrows), bilobed or "butterfly"-shaped nucleus (a horizontal arrow), cerebriform nucleus (an oblique arrow) and "salmon pink"-colored cytoplasm (a vertical arrow) at diagnosis (Wright-Giemsa stain,×1,000).
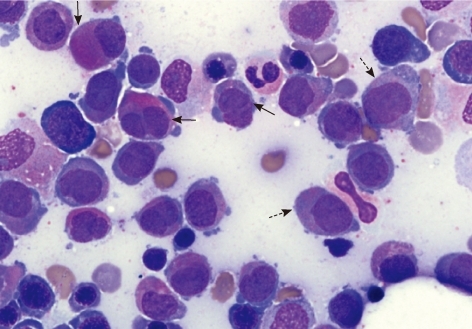
Fig. 2
Chromosome and FISH studies at initial diagnosis. (A) Full karyogram of the bone marrow cells (major clone) at diagnosis: 46,XX,del(6)(?q21q25),der(15)t(15;17)(q22;q12),ider(17)(q10)t(15;17). The arrows indicate abnormal chromosomes in this karyogram. (B) Partial karyograms (chromosomes 15 and 17) of the bone marrow cells at diagnosis: Upper image: t(15;17)(q22;q12) associated with ider(17)(q10)t(15;17). Lower image: t(15;17)(q22;q12) associated with double ider(17)(q10)t(15;17). (C) FISH study using a PML-RARA dual-color, dual-fusion translocation probe (Abbott Molecular/Vysis, USA) at diagnosis. The arrows indicate the PML-RARA or RARA-PML fusion signals. Left image: ider(17)(q10)t(15;17) clone (3 fusion signals). Right image: double ider(17)(q10)t(15;17) clone (5 fusion signals).
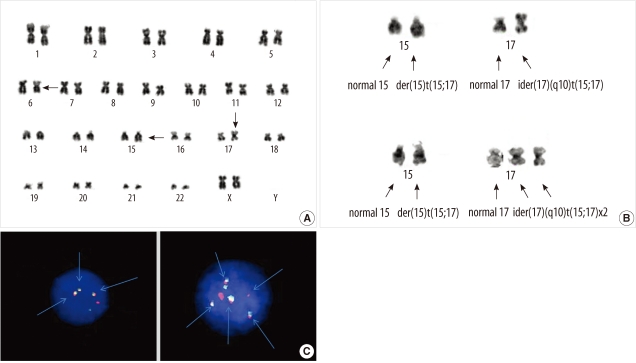
Table 1
Comparison between previous 2 reports and present study with microgranular variant acute promyelocytic leukemia associated with ider(17)(q10)t(15;17)(q22;q12)
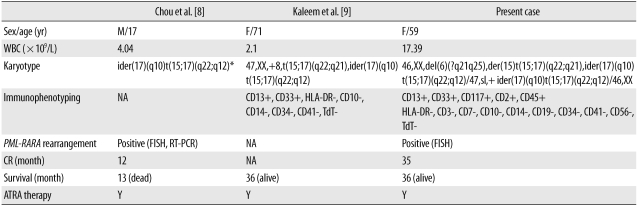
*Full karyotype was not available and published karyotype was slightly modified to simplify nomenclature.
Abbreviations: M, male; F, female; WBC, white blood cell; NA, not available; FISH, fluorescent in situ hybridization; RT-PCR, reverse transcriptase-PCR; CR, complete remission; ATRA, all trans retinoic acid; Y, yes.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download