Abstract
We report a rare case of multiple myeloma with biclonal gammopathy (IgG kappa and IgA lambda type) in a 58-year-old man with prostate cancer who presented with lower back pain. Through computed tomography (CT) imaging, an osteolytic lesion at the L3 vertebra and an enhancing lesion of the prostate gland with multiple lymphadenopathies were found. In the whole body positron emission tomography-computed tomography (PET-CT), an additional osteoblastic bone lesion was found in the left ischial bone. A prostate biopsy was performed, and adenocarcinoma was confirmed. Decompression surgery of the L3 vertebra was conducted, and the pathologic result indicated that the lesion was a plasma cell neoplasm. Immunofixation electrophoresis showed the presence of biclonal gammopathy (IgG kappa and IgA lambda). Bone marrow plasma cells (CD138 positive cells) comprised 7.2% of nucleated cells and showed kappa positivity. We started radiation therapy for the L3 vertebra lesion, with a total dose of 3,940 cGy, and androgen deprivation therapy as treatment for the prostate cancer.
Multiple myeloma is a malignant disease of plasma cells that manifests as disease in the bone marrow, monoclonal protein in the blood and/or urine, and evidence of end organ damage that can be attributed to the underlying plasma cell proliferative disorder [1]. Biclonal gammopathies are a group of disorders characterized by the production of 2 distinct monoclonal proteins. The presence of 2 monoclonal proteins may be because of the proliferation of 2 clones of plasma cells, each producing an unrelated monoclonal immunoglobulin, or it may result from the production of 2 monoclonal proteins by a single clone of plasma cells [2]. Although there are some reports of synchronous occurrence of multiple myeloma and prostate cancer [3, 4], there are no reports of multiple myeloma with biclonal gammopathy accompanied by prostate cancer. Here, we report a rare case of multiple myeloma with biclonal gammopathy accompanied by prostate cancer, which was treated successfully by androgen deprivation therapy for the prostate cancer and surgery combined with radiation therapy for the plasma cell neoplasm.
A 58-yr-old man visited the orthopedic surgery department of a local clinic in January 2008, with a 10-day history of lower back pain and tingling sensation of the left gluteal region and thigh. At the local clinic, spinal magnetic resonance imaging (MRI) was performed, and the clinician suspected a bone tumor. The patient was then transferred to the Hematology/Oncology Department of Eulji University Hospital for further evaluation. The patient had no history of trauma. In addition to the back pain and tingling sensation of the gluteal region and thigh, the patient complained of urinary symptoms, including hesitancy, mild voiding difficulties, and residual urine sensation. A physical examination revealed lower back tenderness, but other findings were normal.
The patient's peripheral white blood cell count was 3,990/µL, hemoglobin and platelet counts were 12.5 g/dL and 225,000/µL, respectively. Blood chemistry tests yielded the following results: total protein, 7.2 g/dL; albumin, 4.3 g/dL; AST, 25 U/L; ALT, 43 U/L; alkaline phosphatase, 88 U/L; total bilirubin, 0.6 mg/dL; calcium, 9.1 mg/dL; phosphorus, 4.3 mg/dL; blood urea nitrogen (BUN), 21 mg/dL; and creatinine, 1.0 mg/dL. Blood electrolyte analysis yielded the following results:sodium, 139 mmol/L; potassium, 4.1 mmol/L; and chloride, 103 mmol/L. Tumor markers detected included alpha-fetoprotein (AFP), 8.1 ng/mL; carcinoembryonic antigen (CEA), 1.49 ng/mL; carbohydrate antigen 19-9 (CA 19-9), 21.29 IU/mL; and prostate specific antigen (PSA), 62.40 ng/mL.
Spinal MRI revealed an osteolytic extension lesion with cortical pinning on the left half of the L3 vertebra, including the left transverse process (Fig. 1). Abdominal and chest computed tomography (CT) revealed an enhancing lesion of the prostate gland. Multiple metastatic lymphadenopathies were discovered in the paraaortic, aortocaval, and common iliac lymph nodes as well as in the left pelvic wall. A whole body positron emission tomography-computed tomography (PET-CT) was performed (Fig. 2A). In the prostate gland, the standardized uptake value (SUV) was 3.45 for the lesion showing fructose-1,6-bisphosphate (FDP) uptake; maximum SUV was 4.57 for the lesion showing FDP uptake in the L3 vertebral body and transverse process; and SUV was 2.87 for the lesion showing FDP uptake in the left iliac bone. Multiple lesions showing FDP uptake were observed in the paraaortic, aortocaval, prevertebral, and left common iliac regions, with an SUV range of 2.27-4.1.
A prostate biopsy was performed under transrectal ultrasonographic guidance, and adenocarcinoma was confirmed in the pathologic review (Fig. 3A). Because of the patient's severe back pain, decompression surgery of the L3 vertebra and a biopsy of the lesion were performed. The biopsy results characterized the lesion as a plasma cell neoplasm (Fig. 3B).
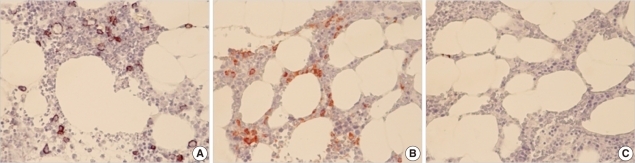
Blood analysis yielded the following values: serum beta 2 microglobulin, 0.17 mg/dL; serum IgG, 1,436.6 mg/dL (reference interval: 870-1,700 mg/dL); IgA, 445.9 mg/dL (reference interval: 110-410 mg/dL); and IgM, 123.62 mg/dL (reference interval: 35-220 mg/dL). Serum protein electrophoresis showed an M-peak in the gamma fraction (serum M-protein was 217 mg/dL), and immunofixation electrophoresis revealed the presence of biclonal gammopathy (IgG kappa and IgA lambda). Urine immunofixation electrophoresis showed a dark band for kappa antisera (Fig. 4). In the 24-hr urine samples, the total protein was 151.2 mg/day and urine protein electrophoresis indicated tubular proteinuria with Bence-Jones proteinuria. Immunohistochemical staining of the bone marrow was performed with CD138, kappa, and lambda, and the bone marrow was positive for CD138 and kappa staining. Bone marrow plasma cells (CD138 positive cells) comprised 7.2% of nucleated cells (Fig. 5). Plain radiographic examination of the whole body did not show any abnormalities other than that in the L3 vertebra. Finally, we diagnosed the patient with multiple myeloma showing biclonal gammopathy accompanied by stage IV prostate cancer (due to an ischial bone metastatic lesion).
We started radiation therapy on the L3 vertebra plasma cell neoplasm, with a total dose of 3,940 cGy, and androgen deprivation therapy with bicalutamide (50 mg/day) and goserelin (3.78 mg/month) as well as bisphosphonate (90 mg/month) for prostate cancer treatment. After 27 months, we performed a whole-body PET-CT (Fig. 2B), which revealed no abnormal FDP uptake in the intra-abdominal lymph nodes, prostate gland, and left iliac bone. Left vertebral body SUV uptake increased to SUV 3.10, but this change was considered as a post-radiotherapy change. PSA decreased to 0.05 ng/mL, which was within the reference interval. Follow-up serum protein electrophoresis revealed an M-peak (serum M-protein was 260 mg/dL) and immunofixation electrophoresis revealed the presence of biclonal gammopathy (IgG kappa and IgA lambda). The patient was administered 10 mg amitriptyline to control the neurologic pain in the left thigh and gluteal region. Nineteen months later, the neurologic pain disappeared, and medication was discontinued. The patient is doing well without evidence of tumor recurrence at 37 months after the initial diagnosis and treatment.
We reported a rare case of multiple myeloma with biclonality (IgG kappa and IgA lambda monoclonal proteins) in a 58-year-old man diagnosed with prostate cancer. The incidence of the simultaneous occurrence of prostate cancer and hematolymphoid malignancies has been reported to be 1.2% [5]. Biclonal gammopathy accounts for approximately 1% of monoclonal gammopathies [2]. To the best of our knowledge, this is the first report of simultaneous prostate cancer and multiple myeloma with biclonal gammopathy.
We initially had difficulty in determining the correct diagnosis of multiple myeloma in this patient. The diagnosis of myeloma requires 1) 10% or more clonal plasma cells on bone marrow examination or biopsy-proven plasmacytoma, 2) presence of serum and/or urinary monoclonal protein (except in patients with true nonsecretory multiple myeloma), and 3) evidence of end-organ damage (hypercalcemia, renal insufficiency, anemia, or bone lesions) related to the underlying plasma cell disorder [1]. In this case, bone marrow plasma cell distribution was 7.2%, and there was no evidence of end organ damage such as anemia, hypercalcemia, and renal insufficiency, except for a single L3 vertebral osteolytic plasma cell neoplasm. Furthermore, the amount of serum M-protein was very small (217 mg/dL). In addition, we performed immunohistochemical staining of bone marrow specimens for CD138, kappa, and lambda and confirmed positive kappa staining. These findings are indicative of bone marrow plasma cell clonality, and therefore, we diagnosed the patient with multiple myeloma.
In many cases, serum electrophoresis produces only a single band on the acetate strip, and biclonal gammopathy is not recognized until immunoelectrophoresis or immunofixation are done. Studies have shown that the clinical features and therapeutic responses of biclonal gammopathy were similar to those of monoclonal gammopathy [2, 6].
In this case, bone marrow plasma cells showed only positive kappa staining and urine immunofixation also showed a single dark band for kappa antisera. The pathogenesis of biclonal gammopathy is unknown, but several potentially related environmental factors have been identified. Biclonal gammopathy may be due to the proliferation of 2 clones of plasma cells, each producing an unrelated monoclonal immunoglobulin, or it may result from the production of 2 monoclonal proteins by a single clone of plasma cells [7]. Unfortunately, we did not conduct a FISH or chromosome study. If these studies had been performed, more information about plasma cell clonality might have been obtained. Monoclonal gammopathies occur in approximately 1-3% of the normal population [2]. Thus, in the present case, it could be possible that the IgA lambda monoclonal protein originated from another cell clone that was unrelated to myeloma.
We do not know whether these 2 diseases, multiple myeloma and prostate cancer, occurred independently or if one disease influenced the development of the other. There are only a few reports of multiple myeloma or monoclonal gammopathy accompanied by prostate cancer [3, 4, 8, 9]. Kao et al. [3] suggested the possible impact of immunosuppression from multiple myeloma and chemokines, including insulin-like growth factor-1 (IGF-1), interleukin-6 (IL-6), stromal cell-derived factor-1 (SDF-1), and vascular endothelial growth factor (VEGF), released by circulating myeloma cells on the progression of prostate cancer. Kahr et al. [8] reported a patient with testicular plasmacytoma after chemical castration for prostate cancer. They suggested that surgical stress may have exacerbated the clinical course of the myeloma, partly because of elevated IL-6 levels after surgery, which would stimulate the growth of myelomas. Kyle and Lust [9] have also reported that repeated antigenic stimulation of the reticuloendothelial system, genetic susceptibility for the development of plasma cell dyscrasia in patients with a positive family history, Epstein-Barr virus infection, lymphoid growth factors (such as IL-6), impairment of T-cell function, and lack of suppression of B cells by T cells play a role in the development of gammopathy. Most studies suggested that immune mechanisms may affect the development of other malignancies, but none of these mechanisms were clearly identified.
In summary, we report a rare case of multiple myeloma with biclonal gammopathy accompanied by prostate cancer. In addition to its rarity, this case may provide some insight into the pathogenesis of plasma cell disorder and prostate cancer.
References
1. Rajkumar SV. Multiple myeloma: 2011 update on diagnosis, risk-stratification, and management. Am J Hematol. 2011; 86:57–65. PMID: 21181954.

2. Kyle RA, Robinson RA, Katzmann JA. The clinical aspects of biclonal gammopathies. Review of 57 cases. Am J Med. 1981; 71:999–1008. PMID: 6797297.
3. Kao J, Jani AB, Vijayakumar S. Is there an association between multiple myeloma and prostate cancer? Med Hypotheses. 2004; 63:226–231. PMID: 15236779.

4. Huang E, Teh BS, Saleem A, Butler EB. Recurrence of prostate adenocarcinoma presenting with multiple myeloma simulating skeletal metastases of prostate adenocarcinoma. Urology. 2002; 60:1111. PMID: 12475684.

5. Terris MK, Hausdorff J, Freiha FS. Hematolymphoid malignancies diagnosed at the time of radical prostatectomy. J Urol. 1997; 158:1457–1459. PMID: 9302142.

6. Knobel D, Zouhair A, Tsang RW, Poortmans P, Belkacémi Y, Bolla M, et al. Prognostic factors in solitary plasmacytoma of the bone: a multicenter Rare Cancer Network study. BMC Cancer. 2006; 6:118. PMID: 16677383.

7. Mahto M, Balakrishnan P, Koner BC, Lali P, Mishra TK, Saxena A. Rare case of biclonal gammopathy. IJCRI. 2011; 2:11–14.

8. Kahr WH, Al-Homadhi A, Meharchand J, Bailey DJ, Stewart AK. Testicular plasmacytoma following chemical orchiectomy: potential role of hypogonadism in myeloma proliferation. Leuk Lymphoma. 1998; 28:437–442. PMID: 9517517.
9. Kyle RA, Lust JA. The monoclonal gammopathies (paraproteins). Adv Clin Chem. 1990; 28:145–218. PMID: 2077876.

Fig. 1
Spinal magnetic resonance imaging (MRI). A plasma cell neoplasm compressing the spinal cord is seen in the L3 vertebral body. (A) Sagittal view. (B) Transverse view.
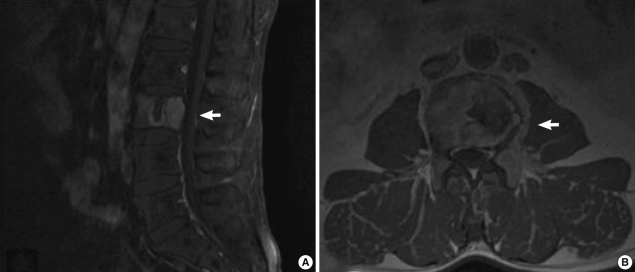
Fig. 2
PET-CT performed at diagnosis and follow-up at 27 months. (A) At diagnosis, increased FDP uptake was seen in the L3 vertebral body, the prostate gland, and ischial bone. Further, hypermetabolic activity was seen in the paraaortic, aortocaval, prevertebral, and left common iliac lymph nodes. These findings may suggest metastatic lymphadenopathies. Increased FDP uptake was seen in the ischial bone. (B) Follow-up PET-CT after 27 months. At the end of the follow-up, an improvement in intra-abdominal lymphadenopathies and disappearance of the hypermetabolic lesion in the prostate gland and ischial bone were observed.
Abbreviations: PET-CT, positron emission tomography-computed tomography; FDP, fructose-1,6-bisphosphate.
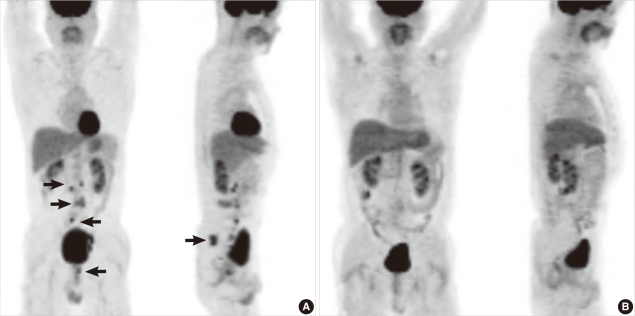
Fig. 3
(A) Prostate gland biopsy. It shows cancer infiltration of individual cells. It may suggest the presence of a gland-forming adenocarcinoma (Hematoxylin & Eosin, ×200). (B) L3 vertebral body biopsy. Some plasma cells show increased nuclear/cytoplasmic ratios, and they contain an eccentric nucleus and a peri-nuclear halo. Some other cells have double nuclei (Hematoxylin & Eosin, ×400).
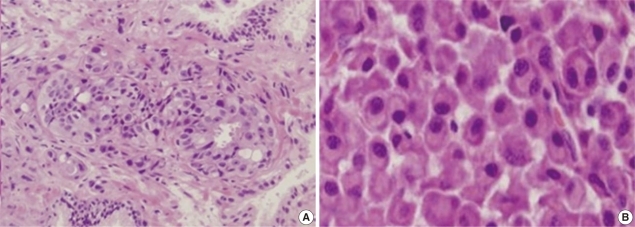
Fig. 4
(A) Protein electrophoresis. An M-peak is seen in the gamma fraction. (B) Serum immunofixation electrophoresis. It shows biclonal gammopathy of IgG kappa type and IgA lambda type. (C) Urine immunofixation electrophoresis. It shows a dark band for kappa antisera.
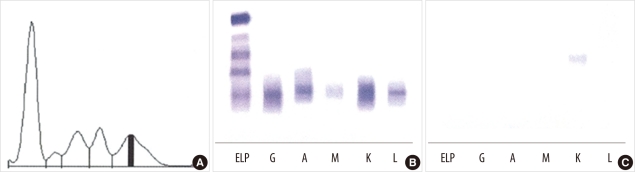
Fig. 5
Bone marrow immunohistochemical staining. (A) CD138 staining. About 7.2% of the marrow nucleated cells showed CD138 immunoreactivity. (B) Kappa staining. Positive staining of kappa light chains is shown. (C) Lambda staining. Negative staining of lambda light chains is shown.
Abbreviation: CD, cluster of differentiation.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download