This article has been
cited by other articles in ScienceCentral.
Abstract
Background
We evaluated the performance of multiplex tandem mass spectrometry (MS/MS) in newborn screening for detection of 6 lysosomal storage disorders (LSDs), namely, Niemann-Pick A/B, Krabbe, Gaucher, Fabry, and Pompe diseases and Hurler syndrome.
Methods
We revised the conditions and procedures of multiplex enzyme assay for the MS/MS analysis and determined the precision of our enzyme assay and the effects of sample amounts and incubation time on the results. We also measured the degree of correlation between the enzyme activities in the dried blood spots (DBSs) and those in the leukocytes. DBSs of 211 normal newborns and 13 newborns with various LSDs were analyzed using our revised methods.
Results
The intra- and inter-assay precisions were 2.9-18.7% and 8.1-18.1%, respectively. The amount of product obtained was proportional to the DBS eluate volume, but a slight flattening was observed in the product vs. sample volume curve at higher sample volumes. For each enzyme assay, the amount of product obtained increased linearly with the incubation period (range, 0-24 hr). Passing and Bablok regression analysis revealed that the enzyme activities in the DBSs and those in the leukocytes were favorably correlated. The enzyme activities measured in the DBSs were consistently lower in patients with LSDs than in normal newborns.
Conclusions
The performance of our revised techniques for MS/MS detection and enzyme assays was of the generally acceptable standard. To our knowledge, this is the first report on the use of MS/MS for newborn screening of LSDs in an Asian population.
Go to :

Keywords: Lysosomal storage disorders, Multiplex enzyme assay, Tandem mass spectrometry, Newborn screening
INTRODUCTION
A lysosome is an intracellular organelle containing numerous acid hydrolases that degrade biological molecules such as proteins, glycoproteins, proteoglycans, lipids, and other complex macromolecules. Lysosomal storage disorders (LSDs) are caused by loss-of-function mutations in the genes encoding for lysosomal hydrolases; these mutations lead to the accumulation of intermediate metabolic products [
1]. More than 40 different LSDs are known, and the incidence of all LSDs is about 1:7,000-1:9,000 [
2]. LSDs are usually diagnosed by performing assays for the enzyme of interest by using an artificial substrate with a fluorescent tag such as 4-methylumbelliferone.
The use of tandem mass spectrometry (MS/MS) in newborn screening programs has improved the detection of inborn errors of metabolism, and this technique can be applied to a wide range of metabolites [
3-
6]. MS/MS screening for LSDs was first described by Li et al. [
7]; they performed direct multiplex assays for Fabry, Gaucher, Krabbe, Niemann-Pick A/B, and Pompe diseases by using dried blood spots (DBS). A refined method for high-throughput analysis by using MS/MS at a newborn screening laboratory was reported by Zhang et al. [
8]. The findings of these studies demonstrated the usefulness of MS/MS in newborn screening for LSDs.
Mucopolysaccharidoses (MPSs) are a group of LSDs. In individuals with MPS, deficiency or malfunction of specific lysosomal enzymes leads to an abnormal accumulation of certain complex carbohydrates such as mucopolysaccharides or glycosaminoglycans. MPSs have several different types and subtypes. Since the symptoms of MPS may not be recognized early in life, the diagnosis of MPS is challenging, and its early detection is necessary. MS/MS techniques for detecting MPS on the basis of DBSs have been developed for some MPSs such as Hurler syndrome (MPS I), Hunter syndrome (MPS II), Maroteaux-Lamy syndrome (MPS VI), and Morquio syndrome type A (MPS IVA) [
9-
15].
In this study, we performed a revised multiplex MS/MS technique for newborn screening of 6 LSDs, namely, Niemann-Pick A/B, Krabbe, Gaucher, Fabry, and Pompe diseases, and MPS I. Although several cases of LSDs in the Korean population have been reported, the incidence and prevalence of LSDs in Korea is unknown. To our knowledge, this is the first report on the use of MS/MS for newborn screening of LSDs in a Korean population. Further, we evaluated the potential of our revised techniques for future use in newborn screening programs.
Go to :

MATERIALS AND METHODS
1. Materials
HPLC-grade methanol (Burdick & Jackson, Muskegon, MI, USA) and water (Mallinckrodt Baker, Phillipsburg, NJ, USA) were used. The enzymes, substrates (S), and internal standards (IS) used to test for various diseases were as follows: acid sphingomyelinase (ASM), ASM-S, and ASM-IS for Niemann-Pick A/B disease; galactocerebrosidase, GALC-S, and GALC-IS for Krabbe disease; acid β-glucocerebrosidase (ABG), ABG-S, and ABG-IS for Gaucher disease; acid α-galactosidase (GLA), GLA-S, and GLA-IS for Fabry disease; acid α-glucosidase (GAA), GAA-S, and GAA-IS for Pompe disease; and α-L-iduronidase (IDU), IDU-S, and IDU-IS for MPS I. All reagents were kindly donated by Genzyme Pharmaceuticals (Cambridge, MA, USA) through the Centers for Disease Control and Prevention (CDC, USA). All the other reagents were of research grade or better quality and were purchased from Sigma Chemical Co. (St. Louis, MO, USA). This research was approved by the Institutional Review Board of the Seoul National University Bundang Hospital.
2. Enzyme assay procedures
All the procedures, including those for preparing the assay buffer and assay cocktail and for conducting the enzyme assay, were performed after modifying the methods developed by Zhang et al. [
8] and Duffey et al. [
12]. For preparing the GAA assay cocktail, 1.8 mL of a 100 g/L solution of 3-[(3-cholamidopropyl) dimethylammonio]-1-propanesulfonate in water, 15.9 mL of 0.3 mol/L citrate-phosphate buffer, and 0.3 mL of a 8 mmol/L solution of acarbose in water were added to a vial containing GAA-S/IS. The GLA assay cocktail was prepared by adding 0.45 mL of a 120 g/L solution of sodium taurocholate in water, 14.67 mL of 0.174 mol/L sodium acetate buffer, and 2.88 mL of 1 mol/L solution of
N-acetylgalactosamine in water to a vial containing GLA-S/IS. The ABG assay cocktail consisted of 2.4 mL of a 120 g/L solution of sodium taurocholate in water and 15.6 mL of 0.62 mol/L citrate-phosphate buffer in a vial containing ABG-S/IS. For preparing the ASM assay cocktail, 0.15 mL of a 120 g/L solution of sodium taurocholate in water, 17.85 mL of 0.92 mol/L sodium acetate buffer, and 0.151 mL of 0.1 mol/L zinc chloride were added to a vial containing ASM-S/IS. The GALC assay cocktail was prepared by adding 1.8 mL of a solution containing 96 g/L sodium taurocholate with 12 g/L oleic acid in water and 16.2 mL of 0.18 mol/L citrate-phosphate buffer to a vial containing GALC-S/IS. For preparing the IDU assay cocktail, 9.9 mL of 0.1 mol/L ammonium formate buffer was added to a vial containing IDU-S/IS.
We punched out 2 DBS disks of 3.2-mm diameter (containing approximately 6.4 µL of dried blood) from each card. One disk was placed in a 96-well plate for the GALC assay, and the other disk was placed on a plate containing 70 µL of sodium phosphate elution buffer and then mixed on an orbital shaker for 1 hr at 250 rpm and 37℃. Each enzyme cocktail (15 µL), except the GALC assay cocktail, was added to a separate plate, and 10 µL of this DBS extract was added to it. The GALC assay cocktail (30 µL) was added to the GALC assay plate. All the plates were incubated on an orbital shaker at 225 rpm and 37℃ for 15 hr. After the reactions were complete, they were quenched with 100 µL of ethyl acetate-methanol (1:1) solution. We used ethyl acetate within 1 week of opening the reagent container. Next, we added 400 µL of both ethyl acetate and water to the plate, transferred 300 µL of the top organic layer to a deep-well plate, and then dried this plate under N2 gas. The dried extract was resuspended in a 100 µL of ethyl acetate-methanol (19:1) solution. Next, 100 mg of silica gel was loaded in the well of the plate, and 250 µL of ethyl acetate-methanol (19:1) solution was added for washing. The resuspended extract was then poured on a filter plate, and it was washed thrice with 400 µL of ethyl acetate-methanol (19:1) solution by using a microplate vacuum filtration apparatus. After drying the plate under N2 gas, the extract was resuspended in 100 µL of the mobile phase (80% acetonitrile, 20% water containing 0.2% formic acid) for MS.
3. Electrospray ionization-MS/MS
Electrospray ionization (ESI)-MS/MS was performed using a Waters Quattro Premier XE tandem mass spectrometer (Waters, MA, USA) in a positive-ion, multiple-reaction monitoring mode and by flow injection [
8]. We injected 10 µL of the resuspended sample into the Waters ACQUITY ultra-performance liquid chromatographic system at an injection rate of 0.1 mL/min for 1.10 min and then at a rate of 0.5 mL/min for 0.4 min. The conditions used for the MS/MS are stated in detail in
Table 1.
Table 1
Conditions for tandem mass spectrometry and multiple reaction monitoring (MRM) transitions of the products and internal standards.
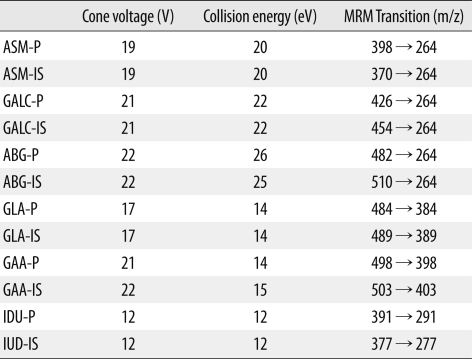

The amount of product was calculated by multiplying the ratio of ion abundance of the product to that of the internal standard (obtained after subtracting the values for the blank from those for the samples) with the amount of the added internal standard and then dividing the value by the ratio of the response factor of the product to that of the internal standard. The response factor was calculated from the calibration curves obtained for the standards containing ratios of product and internal standard. The enzyme activities were expressed in µmol/h/L and were calculated from the amount of product by assuming that a 3.2-mm DBS disk contained 3.2 µL of blood.
4. Determination of precision
Intra-assay precisions (intra-assay CVs) were determined by performing 6 replicated assays for the CDC QC samples at concentrations that showed medium and high activity. To evaluate the inter-assay CVs, enzyme activities of the same CDC samples were measured on 7 consecutive days.
5. Effect of Sample amount and incubation time
To evaluate whether the product obtained in the reaction is linearly related to the amount of enzyme used in the assay, we measured the enzyme activities for 5 µL, 10 µL, and 20 µL of DBSs eluates. Each eluate was analyzed twice. To assess the effect of incubation time, the enzyme reactions were monitored at fixed intervals over 24 hr (0, 4, 8, and 24 hr). Each eluate was analyzed twice.
6. Enzyme activities in DBSs and leukocytes
To evaluate the correlation between the enzyme activities in the DBSs and in the leukocytes, we measured the activity of each enzyme (GALC, ABG, GLA, GAA, and IDU) in both DBSs and leukocytes. Lysosomal enzyme activities in the leukocytes were determined by using appropriate fluorogenic substrates and commonly used methods. Enzyme activities were expressed as nmole of cleaved substrate per milligram of protein per hour at 37℃. Passing and Bablok regression analysis was performed for comparison analysis.
7. DBSs of patients and normal individuals
To validate the capability of our system to detect LSDs in newborns, we analyzed DBSs from 4 patients with Krabbe disease, 1 patient with Gaucher disease, 1 patient with Fabry disease, 6 patients with Pompe disease, and 1 patient with MPS I. All these patients were previously diagnosed to have the respective diseases on the basis of their clinical symptoms and findings of established biochemical tests.
Enzyme activities in the DBSs obtained from 211 newborns without any enzymatic abnormalities were measured as controls.
Go to :

RESULTS
1. Precision
The intra-assay CVs for ASM were 7.7% and 8.5%, for GALC were 4.7% and 2.9%, for ABG were 12.4% and 6.7%, for GLA were 12.0% and 11.6%, for GAA were 6.9% and 5.2%, and for IDU were 18.7% and 8.8% at medium and high concentrations of the enzymes, respectively. Compared to the intra-assay CVs, the inter-assay CVs were greater; the inter-assay CVs for ASM were 9.1% and 16.0%, for GALC were 9.1% and 11.8%, for ABG were 15.4% and 11.1%, for GLA were 18.1% and 16.3%, for GAA were 10.0% and 8.1%, and for IDU were 17.5% and 16.4% at medium and high concentrations of the enzymes, respectively.
2. Effect of sample amount and incubation time
The amount of product obtained was proportional to the volume of the eluate used in the assay, but a slight flattening was observed in the product vs. sample volume curve at higher sample volumes, especially for GALC (
Fig. 1). Zhang et al. [
8] reported similar results, and they suggested that the observed flattening of the curve maybe attributable to the presence of 1 or more inhibitors in the DBSs; an increased eluate volume would lead to an increased inhibitor-to-substrate ratio.
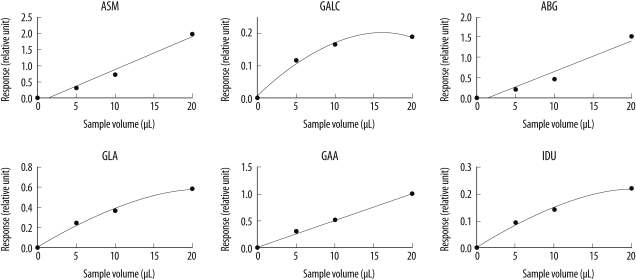 | Fig. 1
Effect of sample amount on enzyme activity. Representative graphs for the 6 enzyme assays with respect to the added volume of DBS eluate.
Abbreviations: ASM, acid sphingomyelinase (Niemann-Pick A/B disease); GALC, galactocerebrosidase (Krabbe disease); ABG, acid β-glucocerebrosidase (Gaucher disease); GLA, acid α-galactosidase (Fabry disease); GAA, acid α-glucosidase (Pompe disease); and IDU, α-L-iduronidase (Hurler disease).

|
In each enzyme assay, the amount of product obtained increased linearly with the incubation period (
Fig. 2). We determined 15 hr of incubation as optimal, and used this incubation time for all subsequent enzyme assays.
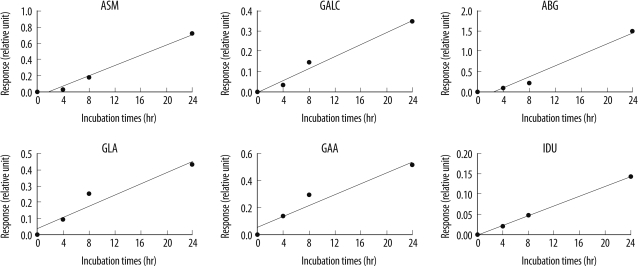 | Fig. 2
Effect of incubation time on enzyme activity. Representative graphs for the time course of the 6 enzyme assays. Each enzyme reaction was monitored for 24 hr.
Abbreviations: ASM, acid sphingomyelinase (Niemann-Pick A/B disease); GALC, galactocerebrosidase (Krabbe disease); ABG, acid β-glucocerebrosidase (Gaucher disease); GLA, acid α-galactosidase (Fabry disease); GAA, acid α-glucosidase (Pompe disease); and IDU, α-L-iduronidase (Hurler disease).

|
3. Enzyme activities in DBSs and leukocytes
Passing and Bablok regression analysis revealed that the enzyme assay in DBSs and leukocytes compared favorably (
Fig. 3).
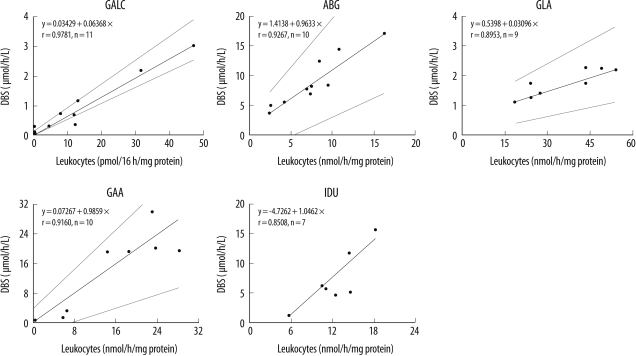 | Fig. 3
Passing and Bablok regression analysis for the lysosomal enzyme activities in the DBSs and leukocytes.
Abbreviations: GALC, galactocerebrosidase (Krabbe disease); ABG, acid β-glucocerebrosidase (Gaucher disease); GLA, acid α-galactosidase (Fabry disease); GAA, acid α-glucosidase (Pompe disease); and IDU, α-L-iduronidase (Hurler disease).

|
4. Enzyme activities in DBSs of patients and normal newborns
The enzyme activities in the DBSs of normal newborns and patients are shown in
Fig. 4. As expected, the enzyme activities in the DBSs of patients were consistently lower than those in the DBSs of normal newborns; the values for the enzyme activities did not overlap. From these findings, we determined the following cut-off points for each enzyme in order to discriminate between normal newborns and patients: 0.45 µmol/h/L for Krabbe disease, 5.74 µmol/h/L for Gaucher disease, 1.31 µmol/h/L for Fabry disease, 2.90 µmol/h/L for Pompe disease, and 7.20 µmol/h/L for MPS I.
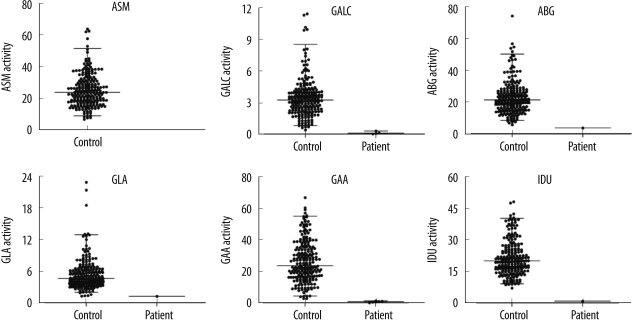 | Fig. 4
Comparison of enzyme activities in DBSs from healthy newborns (n=211) and patients with LSDs (4 patients with Krabbe disease, 1 patient with Gaucher disease, 1 patient with Fabry disease, 6 patients with Pompe disease, and 1 patient with Hurler syndrome).
Abbreviations: ASM, acid sphingomyelinase (Niemann-Pick A/B disease); GALC, galactocerebrosidase (Krabbe disease); ABG, acid β-glucocerebrosidase (Gaucher disease); GLA, acid α-galactosidase (Fabry disease); GAA, acid α-glucosidase (Pompe disease); and IDU, α-L-iduronidase (Hurler disease).

|
Go to :

DISCUSSION
Since the MS/MS techniques were developed for newborn screening of LSDs, several methods for multiplex MS/MS have been introduced; however, the maximum number of diseases reported to be diagnosed by using multiplex MS/MS is 5 [
7,
8]. To our knowledge, this is the first study that reports the feasibility of multiplex MS/MS for diagnosing 6 LSDs, including MPS I, in newborns. The assay performance for our revised method in terms of precision, optimal incubation time, and required sample amounts was of the generally acceptable standard. For each of the 6 enzymes, the assays enabled unambiguous differentiation between samples obtained from healthy newborns and patients (
Fig. 4), suggesting that our method for multiplex MS/MS was effective for newborn screening of LSDs in the Korean population.
A limitation of this study is that only a small number of DBSs were obtained from patients with Krabbe, Pompe, Gaucher, and Fabry diseases and MPS I and that no DBS could be obtained for Niemann-Pick disease leading to a lack of data for this disease. However, we measured the enzyme activities in QC DBSs obtained from the CDC, and our results correlate well with those reported by the CDC (data not shown).
Diagnosis of LSD is usually confirmed by performing an enzyme assay for leukocyte samples by using fluorogenic substrates (such as 4-methylumbelliferone). Therefore, it is important that the enzyme activities measured in the DBSs correlate with those measured in the leukocytes. Although several studies on MS/MS detection of LSD enzymes have been reported [
7-
15], these studies evaluated enzyme activities only in DBSs; to date, no studies on the correlation between the enzyme activities in the DBSs and those in the leukocytes have been performed. We evaluated the correlation between the enzyme activities measured in the DBSs and leukocyte (
Fig. 3) and found a good correlation; this indicates that our screening tests on the DBSs of newborns reflect the original characteristics of the blood sample.
The incidence of LSDs in the Korean population is not known. On the basis of our experience in the field of biochemical diagnosis of LSDs, we think that Krabbe, Gaucher, Pompe, and Fabry diseases and MPS I occur frequently. However, Niemann-Pick A/B disease is rare. Therefore, it is not cost-effective to screen for Niemann-Pick disease in Korea. Other LSDs such as metachromatic leukodystrophy (MLD, defect in arylsulfatase A) and MPS-II (defect in iduronate sulfatase) are relatively common in Korea. Therefore, the diseases included in the group diagnosed by multiplex MS/MS for newborn screening of LSDs in Korea should be changed. We are now developing methods for measuring arylsulfatase A and iduronate sulfatase activities in DBSs by using MS/MS. Our goal is to replace Niemann-Pick disease with MLD and/or MPS-II in the Korean newborn screening program for LSDs.
In conclusion, we evaluated the performance of MS/MS in a newborn screening for LSDs and found the performance to be of the generally acceptable standard. To our knowledge, this is the first report of the use of MS/MS in newborn screening of LSDs in an Asian population.
Go to :

Acknowledgement
This study was supported by grant A090742 received from the Korea Healthcare Technology R&D Project, Ministry for Health & Welfare, Republic of Korea.
Go to :

Notes
Go to :

References
1. Futerman AH, van Meer G. The cell biology of lysosomal storage disorders. Nat Rev Mol Cell Biol. 2004; 5:554–565. PMID:
15232573.

2. Meikle PJ, Hopwood JJ, Clague AE, Carey WF. Prevalence of lysosomal storage disorders. JAMA. 1999; 281:249–254. PMID:
9918480.

3. Chace DH, Kalas TA, Naylor EW. Use of tandem mass spectrometry for multianalyte screening of dried blood specimens from newborns. Clin Chem. 2003; 49:1797–1817. PMID:
14578311.

4. Lehotay DC, Hall P, Lepage J, Eichhorst JC, Etter ML, Greenberg CR. LC-MS/MS progress in newborn screening. Clin Biochem. 2011; 44:21–31. PMID:
20709048.

5. Ko DH, Jun SH, Park HD, Song SH, Park KU, Kim JQ, et al. Multiplex enzyme assay for galactosemia using ultraperformance liquid chromatography-tandem mass spectrometry. Clin Chem. 2010; 56:764–771. PMID:
20299679.

6. Ko DH, Jun SH, Park KU, Song SH, Kim JQ, Song J. Newborn screening for galactosemia by a second-tier multiplex enzyme assay using UPLC-MS/MS in dried blood spots. J Inherit Metab Dis. 2011; 34:409–414. PMID:
21340634.

7. Li Y, Scott CR, Chamoles NA, Ghavami A, Pinto BM, Turecek F, et al. Direct multiplex assay of lysosomal enzymes in dried blood spots for newborn screening. Clin Chem. 2004; 50:1785–1796. PMID:
15292070.

8. Zhang XK, Elbin CS, Chuang WL, Cooper SK, Marashio CA, Beauregard C, et al. Multiplex enzyme assay screening of dried blood spots for lysosomal storage disorders by using tandem mass spectrometry. Clin Chem. 2008; 54:1725–1728. PMID:
18719200.

9. Chamoles NA, Blanco M, Gaggioli D. Diagnosis of alpha-L-iduronidase deficiency in dried blood spots on filter paper: the possibility of newborn diagnosis. Clin Chem. 2001; 47:780–781. PMID:
11274042.
10. Wang D, Eadala B, Sadilek M, Chamoles NA, Turecek F, Scott CR, et al. Tandem mass spectrometric analysis of dried blood spots for screening of mucopolysaccharidosis I in newborns. Clin Chem. 2005; 51:898–900. PMID:
15695324.

11. Blanchard S, Sadilek M, Scott CR, Turecek F, Gelb MH. Tandem mass spectrometry for the direct assay of lysosomal enzymes in dried blood spots: application to screening newborns for mucopolysaccharidosis I. Clin Chem. 2008; 54:2067–2070. PMID:
19042989.

12. Duffey TA, Bellamy G, Elliott S, Fox AC, Glass M, Turecek F, et al. A tandem mass spectrometry triplex assay for the detection of Fabry, Pompe, and mucopolysaccharidosis-I (Hurler). Clin Chem. 2010; 56:1854–1861. PMID:
20940330.

13. Duffey TA, Sadilek M, Scott CR, Turecek F, Gelb MH. Tandem mass spectrometry for the direct assay of lysosomal enzymes in dried blood spots: application to screening newborns for mucopolysaccharidosis VI (Maroteaux-Lamy syndrome). Anal Chem. 2010; 82:9587–9591. PMID:
20961069.

14. Khaliq T, Sadilek M, Scott CR, Turecek F, Gelb MH. Tandem mass spectrometry for the direct assay of lysosomal enzymes in dried blood spots: application to screening newborns for mucopolysaccharidosis IVA. Clin Chem. 2011; 57:128–131. PMID:
21030685.
15. Wolfe BJ, Blanchard S, Sadilek M, Scott CR, Turecek F, Gelb MH. Tandem mass spectrometry for the direct assay of lysosomal enzymes in dried blood spots: application to screening newborns for mucopolysaccharidosis II (Hunter Syndrome). Anal Chem. 2011; 83:1152–1156. PMID:
21192662.

Go to :










 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download