INTRODUCTION
Platelets, the fundamental component of primary hemostasis, are also known to be reservoirs for many growth factors (GFs), which they store in their α-granules. Platelet aggregation and activation, after vascular damage, result in the release of several GFs that may affect the chemotaxis, proliferation, and differentiation of mesenchymal stem cells (MSCs) or other committed cells during the process of tissue repair and healing [
1]. The GFs released from platelets include platelet-derived growth factors (PDGFs), transforming growth factor-beta (TGF-β), vascular endothelial growth factor (VEGF), epidermal growth factor (EGF), fibroblast growth factor (FGF) and insulin-like growth factors (IGFs) [
2,
3].
To explore the possibility that platelet-rich plasma (PRP) could provide an autologous source of these essential GFs that benefit bone and soft tissue healing [
4], many clinical and experimental studies dealing with the effects of PRP have been conducted [
5-
7]. However, the benefit of PRP on bone formation is a controversial subject. While a report suggested a stimulatory effect with the addition of PRP [
5], others have observed no improvement [
6] or have detected even inhibitory effects [
7]. Although the lack of standardization in application across these studies, including differences in the preparation method or dosage of PRP, biomaterials, species, implantation sites, and cell types, may have contributed to the inconsistent results [
8], we hypothesized that the differences in GF composition among PRPs could lead to this discrepancy. GF quantities vary significantly between individuals [
9]. The potential efficacy of PRP depen-ds on the levels of GFs released from platelets; however, no study has yet addressed the relationship between the biologic effects and GF concentrations in PRP. In this study, we attempted to explore the variations in GF release patterns in PRP in order to determine whether differences in GF concentrations influence biologic effects such as proliferation and alkaline phosphatase (ALP) activity of human MSCs (HMSCs) and to identify the factors associated with each of these biologic effects.
Go to :

MATERIALS AND METHODS
This study was reviewed and approved by the Institutional Review Board of Yeungnam University College of Medicine. Prior to drawing blood or bone marrow, informed consent was obtained from each donor.
1. Preparation of PRP and measurement of GF concentrations
First, PRPs were obtained from 3 healthy male volunteers (27 yr, 39 yr, and 43 yr old) to explore the effects of PRP concentration on the proliferation and ALP activity of HMSCs. Then, we obtained donated PRPs from 39 other healthy volunteers to assess the relationship between the effects and GF concentrations. The mean donor age was 42 yr (range, 24-68 yr), distributed at 6-8 individuals per decade. We limited our subjects to those who had not taken anti-platelet medications within 1 week of donation; further, to minimize the possible influences of hormonal variation on the proliferation of HMSCs, we included only male donors.
PRPs were prepared via double centrifugation of blood. In brief, 9 mL of venous blood was drawn into a polypropylene tube containing 1 mL of anticoagulant, acid citrate dextrose (ACD). The blood was centrifuged at 1,500 rpm for 10 min at 25℃ to separate the platelet-containing plasma from the red cells. The platelet-containing plasma was then centrifuged at 3,000 rpm for an additional 10 min at 25℃, and platelet-poor plasma (PPP) was separated out. Platelet pellets were resuspended in 1 mL of plasma and were pooled for PRP. To activate the platelets, 1 part bovine thrombin stock solution (1,000 U/mL; Sigma, St. Louis, MO, USA) was added to 9 parts PRP or PPP to yield a final thrombin concentration of 100 U/mL. Each sample was incubated for 1 hr at 37℃ [
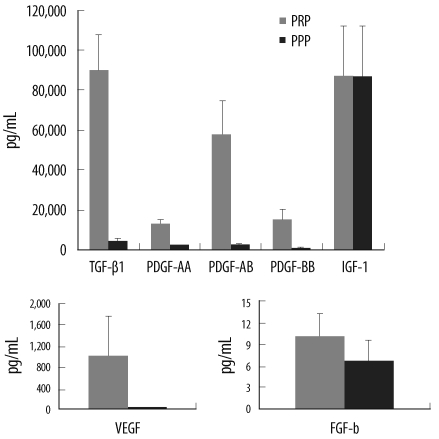
10]. The resulting supernatants from the clot preparation were referred to as activated PRP (aPRP) and activated PPP (aPPP). Both aPRPs and aPPPs were stored at -80℃ until use. The concentrations of PDGF-AA, PDGF-AB, PDGF-BB, TGF-β1, FGF-basic (FGF-b), IGF-1, and VEGF were measured with commercially available Quantikine colorimetric sandwich ELISA kits (R&D Systems, Minneapolis, MN, USA) according to the manufacturer's instructions.
2. Cell isolation
HMSCs were cultured using established protocols [
11]. Bone marrow aspirate was obtained with consent from a 50-yr-old female patient who had undergone an autologous bone graft for the treatment of degenerative spondylolithesis. Mononuclear cells were isolated using a density gradient and were cultured in DMEM (Sigma, St Louis, MO, USA) with 10% fetal bovine serum (FBS), penicillin, and streptomycin (Gibco-BRL, Rockville, MD, USA). The cells were incubated in a humidified atmosphere of 5% CO
2 at 37℃. Non-adherent cells were discarded after 24 hr, and the medium was exchanged every 3 days. First passage (P1) cells were utilized in the study.
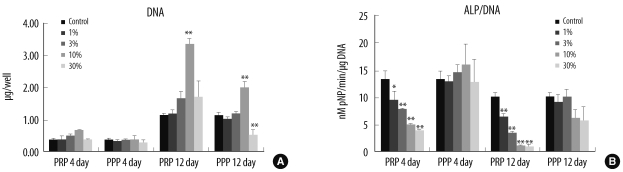
3. The effects of aPRP and aPPP on the DNA content and ALP activity of HMSCs
The cells were seeded at a density of 1.5×10
4 cells/well in 12-well plates (35 mm diameter) and were allowed to attach for 24 hr. After this adhesion period, the medium was removed and was replaced with DMEM with 10% FBS (as control) or variable volume concentrations of aPRP and aPPP (1%, 3%, 10%, and 30%) from each of the 3 donors. Each experiment was conducted in triplicate. Media was exchanged every 4 days. The level of DNA and ALP activity were measured in quadruplicate on the fourth and twelfth days. The DNA level was measured via Hoechst DNA stain, and ALP activity was determined by quantifying the p-nitrophenol released from p-nitrophenol phosphate [
12].
4. Relationships between GF concentrations, DNA content, and ALP activity of HMSCs
In order to evaluate the effects of aPRP GF concentrations on the proliferation or ALP activity of HMSCs, the HMSCs were cultured under the same conditions as described above, in DMEM with 10% aPRPs from each of the 39 donors. After culturing for 12 days, the DNA contents and ALP activity were measured.
5. Statistical analysis
Statistical analysis was conducted using the Predict Analytics Software program (PASW) version 18.0 (SPSS Inc., Chicago, IL, USA). The Shapiro-Wilk test was used to analyze the normal distribution of the data. The effects of aPRP and aPPP concentrations on the DNA content and ALP activity of HMSCs were evaluated via ANOVA, or the Kruskal-Wallis (K-W) test with Bonferroni correction. The differences in GF concentrations between aPRPs and aPPPs were examined using the paired t-test. Pearson's correlation analysis was used to identify correlations between GF levels and age, platelet count, or mean platelet volume. The aPRP release pattern of GFs was grouped via cluster analysis using the K-means method. The relationships between GF concentrations and the biologic effects between these groups was analyzed using ANOVA or K-W test with Bonferroni correction. Stepwise multiple linear regression analysis was employed to show which GFs were associated with DNA content or ALP activity. Significant differences were established at P<0.05; the Bonferroni corrected P value threshold (0.05/number of comparison) was employed for post-hoc analysis of the K-W test.
Go to :

DISCUSSION
Despite 2 decades of clinical study of PRP, little basic data is available regarding the effects of variations in GF levels on biologic function. Because PRP displays the unique characteristics of a mixture of multiple GFs in various concentrations, and because the regenerative potency of PRP probably depends on its GF levels, knowledge of the relationships between GF levels and biologic effects is required to ensure the reliable and reproducible use of PRP [
8]. Therefore, in this study, we attempted to explore the relationship between GF levels and biologic effects.
Obtaining a sufficiently large volume of blood from the single donor in order to analyze the various effects was difficult; thus, we employed DNA analysis for proliferation markers and ALP activity for osteogenic markers of HMSCs. First, we cultured HMSCs at several aPRP concentrations (1%, 3%, 10%, and 30%) from 3 donors to evaluate its effects on proliferation and ALP activity, as well as to determine the optimal concentration in our experimental setting. Simultaneously, HMSCs were cultured with the same concentrations of aPPPs in an effort to demonstrate that the effects were attributable to the presence of platelets. Then, HMSCs were cultured with 10% aPRPs from another 39 donors to assess the relationship between the biologic effects and GF concentrations.
Our findings indicated that aPRP induced HMSC proliferation in a dose-dependent manner. The addition of 10% aPRP to the culture medium induced marked cell proliferation
in vitro; this result was consistent with the findings of previous studies [
13,
14]. Because higher concentration (30%) of aPRP did not promote proliferation, as compared to controls, 10% aPRP may be optimal for the experimental
ex vivo expansion of HMSCs. Some studies also showed that a high concentration of PRP did not promote or that it even suppressed cell proliferation; optimal concentration varied with the cell type or preparation method [
15-
17]. Few studies have suggested the presence of negative regulators in PRP, such as thrombospondin [
17], but the reason for the antiproliferative effect of high-concentration-PRP is not clear. Although aPPP could promote cell proliferation when it is activated, the proliferative effect of aPPP was significantly lower than that of aPRP in this and some other studies. Non-activated PPP cannot induce cell proliferation; therefore, the proliferative effect of aPPP also might be attributed to platelet-derived factors [
16,
18]. However, why higher concentrations of aPPP suppress HMSCs is still unclear.
In the present study, aPRP suppressed the ALP activity of MSCs. Some studies have reported similar results in that PRP increased migration and proliferation, but reduced the osteogenic differentiation of bone marrow-derived MSCs
in vitro [
14,
19]. However, in this study, aPPP did not suppress ALP activity at any concentration; therefore, these inhibitory effects may be associated with substances that are derived from platelets.
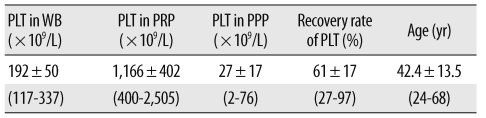
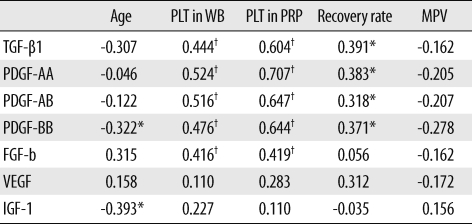
Substantial variations in the PRP and WB platelet counts were noted. The PRP platelet count was correlated with both donor WB platelet counts and recovery rates. The GF concentrations also showed considerable between-donor variations. The aPRP GF concentrations, except for IGF-1, were significantly higher than the aPPP GF concentrations. This suggested that TGF-β1, PDGFs, FGF-b, and VEGF were derived from platelets, but that IGF-1 did not originate from platelets [
20]. The concentrations of TGF-β1, PDGFs, and FGF-b were correlated with the WB and PRP platelet counts, more strongly with the PRP than WB platelet counts. Weibrich et al. [
9] noted that the GFs contents were not well correlated with either the WB or PRP platelet counts; however, this result may be attributed to the use of non-activated PRP in their study. In our study, PDGF-BB and IGF-1 concentration were negatively correlated with age, indicating that GFs vary between individuals according to the differences in platelet count and donor age.
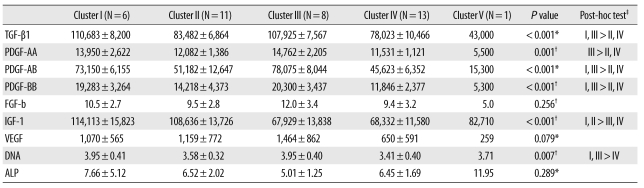
Since the efficacy of PRP is based on the production and release of multiple GFs when platelets are activated, we hypothesized that differing GF concentrations might result in different effects on MSC proliferation or osteogenic differentiation. To verify this hypothesis, HMSCs from 39 donors were cultured with 10% aPRP, and the relationships between GF concentrations and DNA contents or ALP activity were evaluated. The individual variations in PRP GF release patterns were noted, and could be divided into 5 groups based on cluster analysis.
The levels of TGF-β1, PDGFs, and IGF-1 differed between the groups. VEGF and FGF-b displayed tendencies similar to those of TGF-β1 and PDGFs, but these differences were not significant. IGF-1 showed a different tendency, suggesting that IGF-1 originated from a different source than the other GFs. The DNA content of HMSCs is significantly higher in groups with higher TGF-β1 and PDGF contents. These results indicated that individual characteristic release patterns of aPRP GFs may be attributable to GFs derived from platelets and IGF-1 and may influence the different proliferative effects.
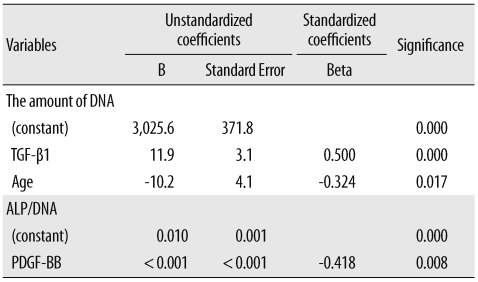
We then performed multiple regression analysis to evaluate independent factors that could affect DNA content or ALP activity. Multiple regression analysis results showed that the quantity of DNA was positively correlated with the concentration of TGF-β1 and was negatively correlated with donor age. This finding indicates that TGF-β1 is the most mitogenic of the aPRP GFs. TGF-β1 has been shown to stimulate the proliferation of undifferentiated MSCs, and its effects vary with the amount applied [
21]. Previous reports have suggested that PDGF-BB and FGF could stimulate the proliferation of MSCs or osteoblastic cells at a concentration of 5 ng/mL [
22,
23] and that the concentrations of these GFs in 10% aPRP were substantially lower than 5 ng/mL. The lack of a detectable effect of PDGF-BB and FGF on MSC proliferation in this study might have been the result of low concentrations of PDGF-BB and FGF that might be present in 10% aPRP.
Although the cellular ALP activity did not differ among groups derived from cluster analysis, aPRP inhibited the ALP activity separately from aPPP. Thus, an unknown substance released from the platelets has been hypothesized to suppress osteogenic differentiation. Multiple regression analysis showed that the ALP activity was negatively correlated with the concentration of PDGF-BB in our study. Tokunaga et al. [
24] noted that the depletion of PDGFR-β in MSCs attenuated mitogenic and migratory responses, but enhanced osteogenic differentiation. Additionally, Ranly reported that PDGF-BB inhibited demineralized bone matrix-induced intramuscular cartilage and bone formation in a dose-dependent manner [
7]. These findings, coupled with the results of our study, indicate that the suppression of ALP activity by aPRP is attributable to PDGF-BB. However, the inhibitory effects of PRP on osteogenesis remain somewhat unclear. The principal disadvantage of this study was that only ALP activity was used as an osteogenic marker, primarily because of the limited available volume of PRP. Kanno et al. [
25] demonstrated that ALP activity was suppressed during cell growth, but was enhanced when the cells achieved confluence. Further studies for optimal duration and the effect on other osteogenic markers, such as osteopontin or RUNX2, should be conducted to demonstrate the osteogenic effects of PRP.
In conclusion, aPRPs stimulate MSC proliferation in a dose dependent manner, and they suppress ALP activity during cell proliferation. The GF concentrations reveal individual variations that may result in different biologic effects of aPRP. MSC proliferation was positively correlated with TGF-β1 concentration, and ALP activity was negatively correlated with PDGF-BB concentration in aPRP. These results indicate that individual differences in GFs should be taken into consideration in order to ensure reliable interpretation of the biologic function and standardized application of PRP.
Go to :





 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download