Abstract
Phaeohyphomycosis is a subcutaneous infection caused by dark pigmented fungi, including fungi of the species Phaeoacremonium, Alternaria, Exophiala, and Pyrenochaeta. In August 2005, a 54-yr-old man who had received a renal transplant 5 yr ago was admitted to our hospital with a subcutaneous mass on the third finger of the right hand; the mass had been present for several months. He had been receiving immunosuppressive agents for several years. He underwent excision of the mass, which was followed by aspiration of the wound for bacterial and fungal cultures. Many fungal hyphae were observed on the histology slide treated with periodic acid-Schiff stain. A few white waxy colonies with a woolly texture grew on the Sabouraud dextrose agar at 30℃ and changed to dark brown in color. Nucleotide sequencing of internal transcribed spacer regions revealed 100% homology to the Phaeoacremonium aleophilum anamorph and Togninia minima teleomorph (514 bp/514 bp). The patient completely recovered after wide surgical excision. Here, we report the first case of phaeohyphomycosis caused by Phaeoacremonium species in a kidney transplant patient in Korea.
The fungal genus Phaeoacremonium was described by Crous et al. [1]. Fungi of this genus have been isolated from a wide range of hosts such as humans, woody plants, and the larvae of bark beetles, but only some species of this genus have been reported as pathogens of humans [2, 3]. Presently, the Phaeoacremonium genus, formerly known as Phialophora, includes more than 20 species [4, 5]. The occurrence of Phaeoacremonium species varies, with Phaeoacremonium aleophilum being the most frequently isolated species in esca diseased grapevines [2].
The term phaeohyphomycosis was first introduced in 1974 to describe local infections caused by darkly pigment-ed molds (black mold) [6, 7]. However, only a few human phaeohyphomycosis cases have been reported in the literature worldwide [8]. We report the first case of primary phaeohyphomycosis by Phaeoacremonium species in a kidney transplant patient in Korea.
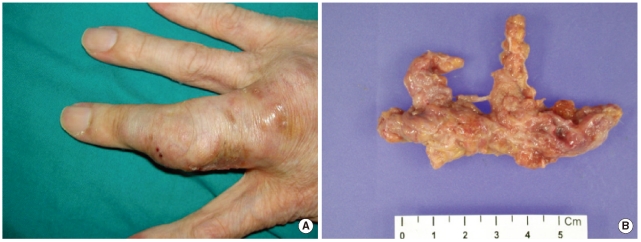
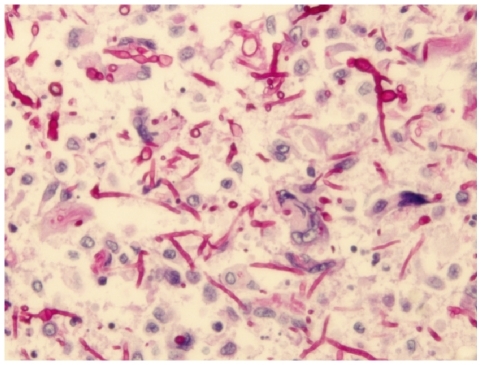
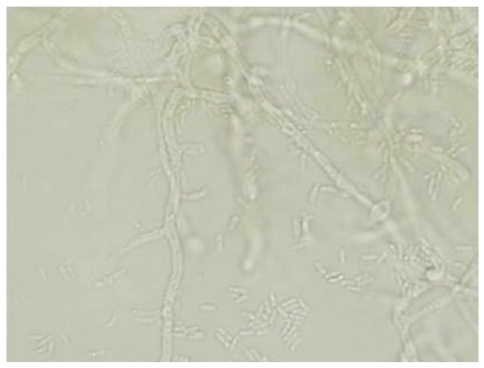
A 54-yr-old man with a large subcutaneous mass on the third finger of his right hand was admitted to our hospital in August 2005 (Fig. 1A). He had been working at a leather factory for 10 yr. He had chronic renal failure caused by diabetes mellitus, and therefore, he underwent kidney transplantation in October 2000. After the operation, he was prescribed immunosuppressive agents such as tacrolimus, deflazacort, and mycophenolate mofetil. He had no history of trauma, but had a small nodule on the third finger since 1995. The nodule grew gradually during several months prior to hospital admission. The patient also experienced mild pain and pruritus around the lesion. The mass was removed by an orthopedic surgeon. The patient had high serum glucose levels of 341 mg/dL, which suggested post-tra-nsplant diabetic mellitus; his serum blood urea nitrogen/creatinine levels were 33.5/1.5 mg/dL. In complete blood count analysis, the white blood cell count and Hb and Hct levels were within reference ranges, although platelet count was slightly low at 117,000/µL. The patient underwent excision of the mass, which was followed by culture of the wound aspirate collected in the operating room. The mass was removed entirely and had dimensions of 6.5×3×1.2 cm (Fig. 1B). In the bacterial culture, no colonies formed on the blood agar, MacConkey agar, or thioglycolate broth after incubation for 48 hr at 35℃ and ambient temperature. Periodic acid-Schiff staining performed on the histology slide showed many fungal hyphae (Fig. 2). Few white waxy colonies with a woolly texture were observed on Sabouraud dextrose agar at 30℃, and the colonies changed into a dark-brown color. When the fungal hyphae were separated on the slide culture, the conidiophores were observed to be short and frequently unbranched. Furthermore, the apical cell of the conidiophores mainly represented 1 phialide. The phialides appeared to be elongate-ampulliform and were long or subcylindrical. This morphology further supported the presence of the genus Phaeoacremonium (Fig. 3). PCR using ITS1 (5' TCC GTA GGT GAA CCT GCG G 3') and ITS4 (5' TCC TCC GCT TAT TGA TAT GC 3') primers was performed for species identification [9]. The β-tubulin and actin genes were detected according to CLSI guidelines, 2008 [10]. Even though β-tubulin and actin were not amplified, nucleotide sequencing of ITS1 and ITS4 revealed 100% sequence homology to both P. aleophilum anamorph and Togninia minima teleomorph. As such, antifungal agents, including fluconazole and amphotericin B, were administered to the patient empirically for 2 days, and the patient recovered without any sequelae. Five years after the wide excision, the patient has had no further subcutaneous lesions.
Phaeohyphomycosis is the term used to describe a subcutaneous fungal infection caused by darkly pigmented molds (black mold), such as those from the species Phaeoacremonium, Phialophora, Alternaria, Exophiala, and Pyrenochaeta [6, 7]. This condition is rare in humans, but it can involve the skin, subcutis, paranasal sinuses, or the central nervous system [9]. The genus Phaeoacremonium has been isolated from patients who experience traumatic implantation of fungi from contaminated plant thorns or soil, and was usually known as a causal agent of grapevine diseases such as Petri disease and esca, which can cause leaf drop and wood discoloration [7, 11]. Recently, cases of phaeohyphomycosis caused by Phaeoacremonium species have been increasingly reported in humans [7, 8]. Notably, these diseases have been described in immunocompromised patie-nts with renal transplantation [12]. In our case, the patient was in an immunosuppressed state. Although he lived in an urban area, he frequently climbed mountains as a hobby before the appearance of the subcutaneous mass. When we consider the natural ecological niches of the Phaeoacremonium species, the patient may have been exposed to this pathogen during his frequent mountain-climbing trips. It is difficult to explain how he acquired the infection because he did not report any injury. However, it is possible that microtraumas to the skin and immunosuppressive therapy may have played a role.
Morphologically, the genus Phaeoacremonium is an intermediate between the genera Acremonium and Phialophora [8, 13]. Organisms of the genus Acremonium usually produce delicate hyaline septate hyphae, which carry unbran-ched tapering conidiophores with elliptical-shaped unicellular conidia. The characteristic tapering phialides of these organisms are derived from septate hyphae with one-celled ellipsoidal conidia [14]. In contrast, organisms of genus Phialophora show melanized hyphae and slimy one-celled conidia at their apex with distinct collarettes [15]. Phaeoacremonium show medium brown hyphae, which become pale brown to hyaline and verruculose. The phialides have a funnel-shaped collarette and show a wide variety of diverse forms, including ellipsoidal, obovate, cylindrical, or allantoid (sausage-like) [5]. The morphological distinctions from a number of other relatively similar Phaeoacremonium species have been summarized by Mostert et al. [5]. Although it is difficult to identify the species in our isolate on the basis of morphological criteria, it could be distinguished from species of brown colonies, such as Phaeoacremonium parasiticum and Phaeoacremonium inflatipes, by its short and usually unbranched conidiophores. However, molecular-based tools must be used in order to confirm the species. Although the additional PCRs for β-tubulin and actin were unsuccessfully repeated several times, sequence analysis of internal transcribed spacer (ITS) revealed 100% sequence homology to P. aleophilum and T. minima. T. minima is a teleomorph of P. aleophilum [11]. CLSI guidelines indicate that the ITS region in Phaeoacremonium only provides limited resolution for species identification [10]. Thus, we are able to conclude that the pathogen was of the genus Phaeoacremonium. Optimal treatment of phaeohyphomycosis usually involves surgical wound excision combined with antifungal agents for several months [16]. For this patient, empirical antifungal agents were administered for 2 days and the wound excision was successfully completed. Total excision of the nodule was important for uncomplicated healing in the patient. After 5 yr, no subcutaneous lesion has been recognized in the patient. Herein, we have reported the first case of phaeohyphomycosis caused by the Phaeoacremonium species in a kidney transplant patient in Korea.
References
1. Crous PW, Gams W, Wingfield MJ, Van Wyk PS. Phaeoacremonium gen. nov. associated with wilt and decline diseases of woody hosts and human infections. Mycologia. 1996; 88:786–796.
2. Aroca A, Raposo R, Lunello P. A biomarker for the identification of four Phaeoacremonium species using the beta-tubulin gene as the target sequence. Appl Microbiol Biotechnol. 2008; 80:1131–1140. PMID: 18719899.
3. Aguilar-Donis A, Torres-Guerrero E, Arenas-Guzman R, Hernandez-Hernandez F, Lopez-Garcia L, Criales-Vera S, et al. Mycetoma caused by Phaeoacremonium parasiticum- a case confirmed with B-tubulin sequence analysis. Mycoses Epub 2010 Aug 4. http://www.ncbi.nlm.nih.gov/pubmed/20701685.
4. Essakhi S, Mugnai L, Crous PW, Groenewald JZ, Surico G. Molecular and phenotypic characterisation of novel Phaeoacremonium species isolated from esca diseased grapevines. Persoonia. 2008; 21:119–134. PMID: 20396582.
5. Mostert L, Groenewald JZ, Summerbell RC, Robert V, Sutton DA, Padhye AA, et al. Species of Phaeoacremonium associated with infections in humans and environmental reservoirs in infected woody plants. J Clin Microbiol. 2005; 43:1752–1767. PMID: 15814996.
6. Naggie S, Perfect JR. Molds: hyalohyphomycosis, phaeohyphomycosis, and zygomycosis. Clin Chest Med. 2009; 30:337–353. vii–viii. PMID: 19375639.

7. Guarro J, Alves SH, Gene J, Grazziotin NA, Mazzuco R, Dalmagro C, et al. Two cases of subcutaneous infection due to Phaeoacremonium spp. J Clin Microbiol. 2003; 41:1332–1336. PMID: 12624080.
8. Baradkar VP, Mathur M, Kumar S. Phaeohyphomycosis of subcutaneous tissue caused by Phaeoacremonium parasiticum. Indian J Med Microbiol. 2009; 27:66–69. PMID: 19172066.
9. Kumar KK, Hallikeri K. Phaeohyphomycosis. Indian J Pathol Microbiol. 2008; 51:556–558. PMID: 19008596.

10. Clinical and Laboratory Standerds Institute. Interpretive criteria for identification of bacteria and fungi by DNA target sequencing (MM18-A) 2008.
11. Damm U, Mostert L, Crous PW, Fourie PH. Novel Phaeoacremonium species associated with necrotic wood of Prunus trees. Persoonia. 2008; 20:87–102. PMID: 20467488.
12. Farina C, Gotti E, Mouniee D, Boiron P, Goglio A. Phaeoacremonium parasiticum subcutaneous infection in a kidney-transplanted patient successfully treated by surgery. Transpl Infect Dis. 2007; 9:253–255. PMID: 17605749.
13. Reblova M, Seifert KA. A new fungal genus, Teracosphaeria, with a phialophora-like anamorph (Sordariomycetes, Ascomycota). Mycol Res. 2007; 111:287–298. PMID: 17363235.
14. Das S, Saha R, Dar SA, Ramachandran VG. Acremonium species: a review of the etiological agents of emerging hyalohyphomycosis. Mycopathologia. 2010; 170:361–375. PMID: 20577905.
15. de Hoog GS, Mayser P, Haase G, Horre R, Horrevorts AM. A new species, Phialophora europaea, causing superficial infections in humans. Mycoses. 2000; 43:409–416. PMID: 11204358.
16. Larsen CG, Arendrup MC, Krarup E, Pedersen M, Thybo S, Larsen FG. Subcutaneous phaeohyphomycosis in a renal transplant recipient successfully treated with voriconazole. Acta Derm Venereol. 2009; 89:657–658. PMID: 19997708.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download