β-Hemolytic streptococcal isolates obtained from humans can be subdivided into large-colony and small-colony (<0.5 mm in diameter) formers. Large colony formers include
Streptococcus pyogenes (Lancefield group A antigen),
Streptococcus agalactiae (Lancefield group B antigen), and
Streptococcus dysgalactiae subsp.
equisimilis (Lancefield group C and G antigens) [
1]. The small-colony-forming β-hemolytic strains with Lancefield group A, C, F, or G antigens are considered part of the viridans group streptococci (VGS). VGS also include
Streptococcus mitis,
Streptococcus oralis,
Streptococcus mutans,
Streptococcus salivarius,
Streptococcus sanguinis, and
Streptococcus bovis [
1]. Although penicillin remains the drug of choice in the treatment of infections caused by large-colony-forming β-hemolytic streptococci (BHS), drug tolerance and clinical therapeutic failures have been reported [
2]. Macrolides and lincosamides have been frequently used to prevent β-lactam allergies in patients. These agents are also used in empiric and preventive therapies for the treatment of BHS infections [
3,
4]. However, recent studies have shown considerable changes in the susceptibility of BHS to erythromycin and clindamycin, although different resistance rates to these agents owing to geographical variation and investigators have been reported [
5-
7]. β-Lactam agents have been the treatment of choice for VGS infections; however, increase in the incidence of VGS with multidrug-resistance to penicillin and other agents, such as cephalosporins, macrolides, lincosamides, tetracycline, quinupristin-dalfopristin, and quinolones, has been reported [
7,
8]. Moreover, CLSI has recommended that VGS isolated from normally sterile body sites should be tested for penicillin susceptibility by using a minimum inhibitory concentration (MIC) method and interpretive criteria [
9]. Accurate susceptibility testing for BHS and VGS is required in order to guide appropriate antimicrobial therapy and to monitor further spread of resistant pathogens. Rising drug resistance of BHS and VGS has increased the need for accurate determination of antimicrobial susceptibility in a timely manner in clinical microbiology laboratories. Rapid reporting of the results of an antimicrobial susceptibility test (AST) has been shown to improve patient outcomes and reduce hospital costs [
10,
11]. Because there are significant differences in the susceptibility of BHS and VGS to β-lactam agents, there are separate interpretive criteria for the susceptibility of the 2 groups of organisms to ampicillin, penicillin, cefotaxime, ceftriaxone, and cefepime [
9].
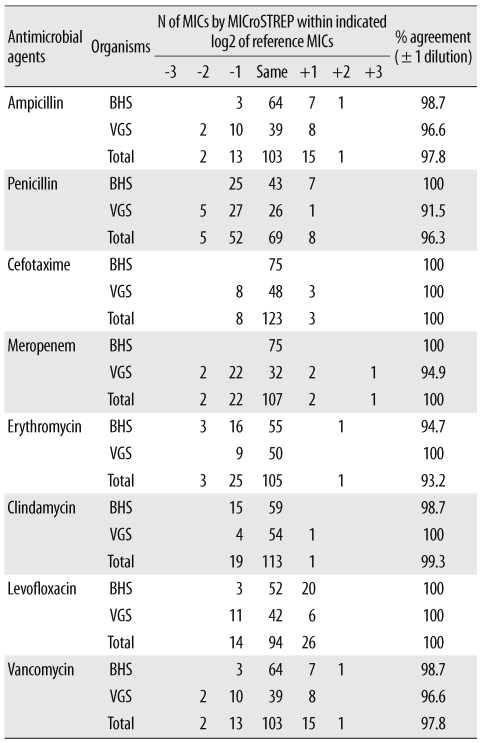
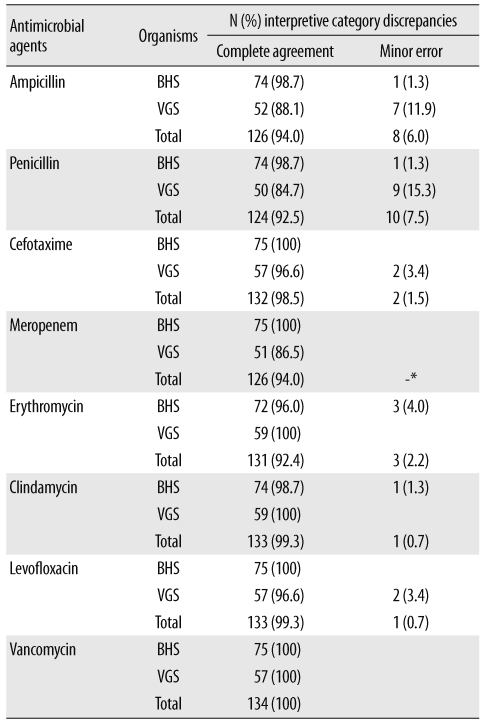
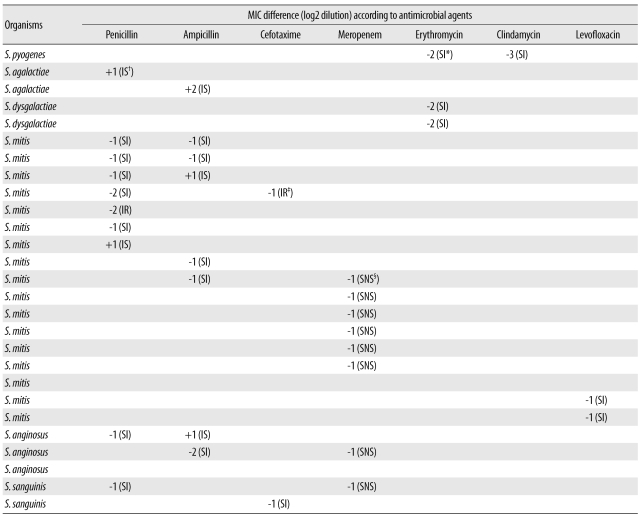
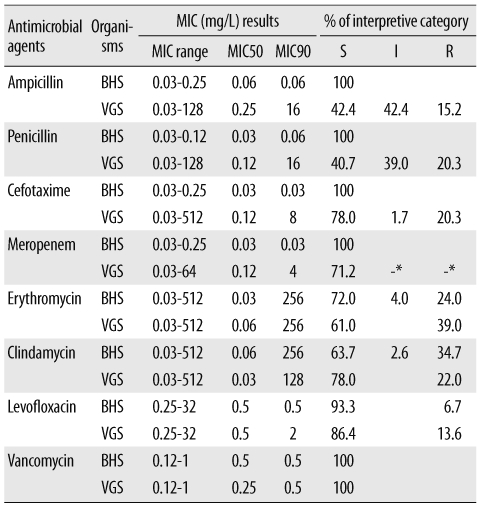
Automated commercial susceptibility test systems for streptococci offer reliable AST results for MIC measurement and help accurately determine the antimicrobial susceptibility profile according to the Streptococcus group. However, most studies are focused on evaluating the AST performance of Streptococcus pneumoniae. To the best of our knowledge, no study has been conducted to evaluate the accuracy of the automated MicroScan (Siemens Healthcare Diagnostics, Sacramento, CA, USA) AST system for susceptibility testing of BHS and VGS. Therefore, this study was designed to evaluate the clinical usefulness of the MicroScan MICroSTREP plus antimicrobial panel (MICroSTREP) as a susceptibility testing system for BHS and VGS.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download