Abstract
Monoclonal gammopathy occurs in one-third of the patients with mucosa-associated lymphoid tissue lymphoma (MALT lymphoma). However, monoclonal gammopathy has been rarely reported in Korea. Paraprotenemia accompanying MALT lymphoma is strongly correlated with involvement of the bone marrow, and this involvement leads to the progression of the disease. Here, we present a case of a 66-yr-old man diagnosed with IgM monoclonal gammopathy and stage IV extranodal marginal zone lymphoma of the small intestine, with the involvement of the bone marrow.
Extranodal marginal zone B-cell lymphoma (ENMZL) of the mucosa-associated lymphoid tissue (MALT) accounts for 7-8% of all newly diagnosed B-cell lymphomas [1]. Usually ENMZL is an indolent disease, but some patients have shown dissemination of ENMZL into the bone marrow [2, 3].
Some studies on monoclonal gammopathy have suggested the potential of ENMZL cells to produce immunoglobulins [4-6]. The prevalence of monoclonal gammopathy in MALT lymphoma patients was reported to be 36% by Wöhrer et al. [7]. They suggested that the change in the paraprotein concentration may reflect an antitumor response. One study described that the presence of a monoclonal spike is strongly correlated with bone marrow involvement, which is indicative of advanced stages of the disease [8].
In Korea, monoclonal gammopathy in MALT lymphoma has been rarely reported until recently [9]. Here, we report a case of a 66-yr-old man diagnosed with IgM monoclonal gammopathy and ENMZL of the small intestine.
A 66-yr-old man was admitted to the hospital because of back pain and dyspnea on exertion. The patient had no history of medical illness, and he had good health until this hospital visit. Complete blood count analysis revealed that the hemoglobin level was 8.6 g/dL with microcytic and hypochromic anemia. The iron panel indicated that his anemia was caused by iron deficiency: the serum ferritin level was 5.62 ng/mL (normal range, 30-310 ng/mL) and transferrin saturation was 7.34% (20-55%). His serum chemical values were as follows: total protein, 7.9 g/dL (normal range, 6.4-8.3 g/dL); albumin, 2.7 g/dL (3.4-5.1 g/dL); lactate dehydrogenase level, 366 IU/L (240-480 IU/L); calcium level, 8.0 mg/dL (8.4-10.0 mg/dL); blood urea nitrogen, 12.7 mg/dL (6.0-20.0 mg/dL); and creatinine level, 0.8 mg/dL (0.5-1.2 mg/dL). Serum protein electrophoresis showed an abnormal zone of restriction in the gamma globin lane. The level of the M-peak was 2.1 g/dL. Serum immunofixation electrophoresis demonstrated monoclonal gammopathy of the IgM lambda type (Fig. 1).
Computed tomography (CT) was performed because the patient developed abrupt severe abdominal pain. The CT scan showed a circumferential huge mass in the ileal loop along with perforation and multiple lymphadenopathy. The patient underwent an emergency operation, and he was diagnosed with ENMZL of MALT with focal large cell transformation (diffuse large B-cell lymphoma) of the small bowel. Histologic examination revealed the MZ tumor cells infiltrating around the reactive follicles and disseminating to the small bowel mucosa (Fig. 2A). The tumor cells were small to medium-sized, and they contained slightly irregular nuclei with moderately dispersed chromatin and inconspicuous nucleoli. The intestinal crypt epithelium was often invaded and destroyed by these tumor cells, leading to the formation of the lymphoepithelial lesion (Fig. 2B). Plasmacytic differentiation was observed, which was verified by immunohistochemistry for CD38 (Fig. 2C, D). Immunohistochemical analysis showed that the neoplastic lymphocytes were positive for CD20 and negative for CD5, CD10, CD23, and cyclin D1.
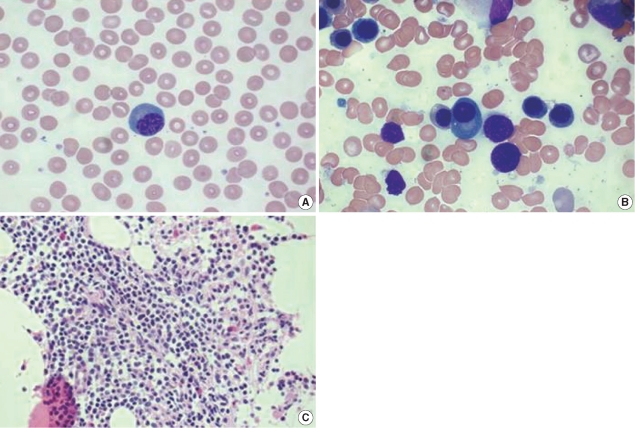
Bone marrow biopsy was performed, which showed bone marrow involvement of ENMZL. The bone marrow aspirate showed 20% cellularity and 2.1% of plasma cells (Fig. 3B). Bone marrow biopsy revealed focal infiltration of the neoplastic lymphoid cells, which showed a morphologic spectrum, including small lymphocytes with inconspicuous nucleoli and plasmacytoid lymphocytes (Fig. 3C).
A whole-body positron emission tomography (PET)-CT scan revealed a hypermetabolic mass (diameter, 7 cm) in the ileum and multiple abdominopelvic lymph node metastases. The patient was diagnosed with IgM monoclonal gammopathy and stage IV ENMZL of the small intestine involving the bone marrow and multiple abdominopelvic lymph nodes. The patient was administered a combination chemotherapy of cyclophosphamide (750 mg/m2 on day 1), adriamycin (50 mg/m2 on day 1), vincristine (1.4 mg/m2 on day 1), prednisolone (100 mg/day on days 1-5), and rituximab (375 mg/m2 on day 1) every 21 days. After the fourth cycle of chemotherapy, the patient recovered, and he is now being followed-up on an outpatient basis.
ENMZL of the MALT accounts for 7-8% of non-Hodgkin's lymphomas (NHL) [1]. ENMZL may occur in the stomach, intestine, orbit, lung, thyroid, salivary gland, skin, soft tissues, bladder, kidney, and central nervous system. The stomach is the most common site of involvement [10]. ENMZL is diagnosed on the basis of morphological examination and immunohistochemistry, including a panel of monoclonal antibodies against CD20, CD79a, CD3, CD10, CD23, CD43, Bcl-2, and cyclin D1, according to the Revised European American Lymphoma (REAL)/WHO classification [11]. ENMZL is regarded as an indolent disease, but it sometimes progresses to a more aggressive histopathogenesis and shows resistance to therapy. For patients who present with an unfavorable International Prognostic Index score and disseminated disease involving extranodal sites, the prognosis is relatively poor [2, 3].
The association of monoclonal gammopathy with B cell NHL is a well-known phenomenon, although the frequency varies between the histological subtypes. Approximately 50% of patients with lymphoplasmacytic lymphoma have elevated serum Ig levels. Paraproteinemias are often noted in patients with CLL and are less common in patients with diffuse large B-cell lymphoma [12]. The presence of monoclonal gammopathy has been reported in up to 50% of patients with splenic MZL, and it has been reported in 36% of those with ENMZL [7, 13].
Asatiani et al. [8] showed that monoclonal gammopathy in patients with ENMZL was associated with bone marrow involvement and a tendency toward large cell transformation. This study showed that the common Ig in monoclonal gammopathy was IgG, while the incidence of IgM was low (3.8%; 1/26). Our case showed bone marrow involvement and transformation from marginal zone B-cell lymphoma to large cell lymphoma in ENMZL.
The association of ENMZL with monoclonal gammopathy has been rarely reported in Korea. One case of gastric MALT lymphoma with monoclonal gammopathy but absence of Helicobacter pylori infection was reported recently in Korea [9]. Monoclonal gammopathy is negatively associated with response to eradication of H. pylori in gastric MALT lymphoma. The effect of monoclonal gammopathy on the treatment response in cases of small intestine MALT lymphoma remains unknown. The effect of changing paraprotein levels on the treatment response was observed: our patient who had a paraprotein level of 2.1-0.8 g/dL after the fourth cycle of chemotherapy showed an antitumor response. Reitter et al. [14] insisted that the paraprotein level should be routinely checked at the staging of MALT lymphoma and suggested that the change in the paraprotein level may be valuable for monitoring the treatment response.
Our report shows a rare case of monoclonal gammopathy in a patient with MALT lymphoma. Further investigations are required to determine whether the routine check for paraprotein level is needed and whether the presence of monoclonal gammopathy can assist in the prognostic stratification of patients with ENMZL.
References
1. A clinical evaluation of the International Lymphoma Study Group classification of non-Hodgkin's lymphoma. The Non-Hodgkin's Lymphoma Classification Project. Blood. 1997; 89:3909–3918. PMID: 9166827.
2. Raderer M, Streubel B, Woehrer S, Puespoek A, Jaeger U, Formanek M, et al. High relapse rate in patients with MALT lymphoma warrants lifelong follow-up. Clin Cancer Res. 2005; 11:3349–3352. PMID: 15867234.
3. Thieblemont C, Berger F, Dumontet C, Moullet I, Bouafia F, Felman P, et al. Mucosa-associated lymphoid tissue lymphoma is a disseminated disease in one third of 158 patients analyzed. Blood. 2000; 95:802–806. PMID: 10648389.

4. Tirelli A, Guastafierro S, Cava B, Lucivero G. Triclonal gammopathy in an extranodal non-Hodgkin lymphoma patient. Am J Hematol. 2003; 73:273–275. PMID: 12879432.

5. Tursi A, Modeo ME. Monoclonal gammopathy of undetermined significance predisposing to Helicobacter pylori-related gastric mucosa-associated lymphoid tissue lymphoma. J Clin Gastroenterol. 2002; 34:147–149. PMID: 11782609.
6. Yamasaki S, Matsushita H, Tanimura S, Nakatani T, Hara S, Endo Y, et al. B-cell lymphoma of mucosa-associated lymphoid tissue of the thymus: a report of two cases with a background of Sjögren's syndrome and monoclonal gammopathy. Hum Pathol. 1998; 29:1021–1024. PMID: 9744322.
7. Wöhrer S, Streubel B, Bartsch R, Chott A, Raderer M. Monoclonal immunoglobulin production is a frequent event in patients with mucosa-associated lymphoid tissue lymphoma. Clin Cancer Res. 2004; 10:7179–7181. PMID: 15534090.

8. Asatiani E, Cohen P, Ozdemirli M, Kessler CM, Mavromatis B, Cheson BD. Monoclonal gammopathy in extranodal marginal zone lymphoma (ENMZL) correlates with advanced disease and bone marrow involvement. Am J Hematol. 2004; 77:144–146. PMID: 15389912.

9. Hwang EK, Jang EJ, Kim SM, Jeong SH, Lee HW, Kang SY, et al. A case report of Helicobacter pylori negative gastric MALT lymphoma with monoclonal gammopathy. Korean J Hematol. 2009; 44:261–267.
10. Ott MM, Rosenwald A, Katzenberger T, Dreyling M, Krumdiek AK, Kalla J, et al. Marginal zone B-cell lymphomas (MZBL) arising at different sites represent different biological entities. Genes Chromosomes Cancer. 2000; 28:380–386. PMID: 10862046.

11. Swerdlow SH, Campo E, Harris NL, Jaffe ES, Pileri SA, Stein H, et al. WHO classification of tumours of haematopoietic and lymphoid tissues. 2008. 4th ed. Lyon: International Agency for Research on Cancer;p. 214–216.
12. Deegan MJ, Abraham JP, Sawdyk M, Van Slyck EJ. High incidence of monoclonal proteins in the serum and urine of chronic lymphocytic leukemia patients. Blood. 1984; 64:1207–1211. PMID: 6437461.

13. Catovsky D, Matutes E. Splenic lymphoma with circulating villous lymphocytes/splenic marginal-zone lymphoma. Semin Hematol. 1999; 36:148–154. PMID: 10319383.
14. Reitter S, Neumeister P, Beham-Schmid C, Emberger W, Strunk D, Brezinschek R, et al. A case of generalized MALT lymphoma with IgM paraproteinemia and peripheral blood involvement. Ann Hematol. 2010; 89:213–214. PMID: 19582453.

Fig. 1
Serum immunofixation electrophoresis showed a dense abnormal zone of restriction in the IgM lane and in the lambda light chain lane.
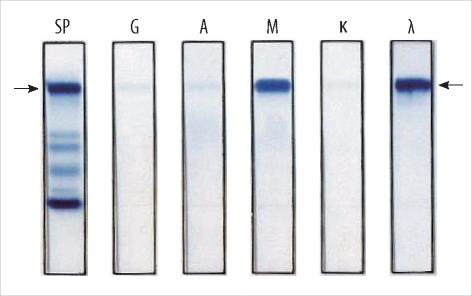
Fig. 2
Microscopic features of the small intestinal mucosa-associated lymphoid tissue lymphoma. (A) The tumor cells of the marginal zone surround the reactive follicles and diffusely infiltrate the mucosa (hematoxylin and eosin stain, ×100). (B) A lymphoepithelial lesion showing destruction of the intestinal crypts by tumor cells (arrows) (hematoxylin and eosin stain, ×400). (C) Plasma cells with eccentric nuclei are admixed with small lymphocytes (hematoxylin and eosin stain, ×400). (D) Plasma cells are highlighted (brown) by immunohistochemical staining for CD38 (Immunohistochemistry, ×400).
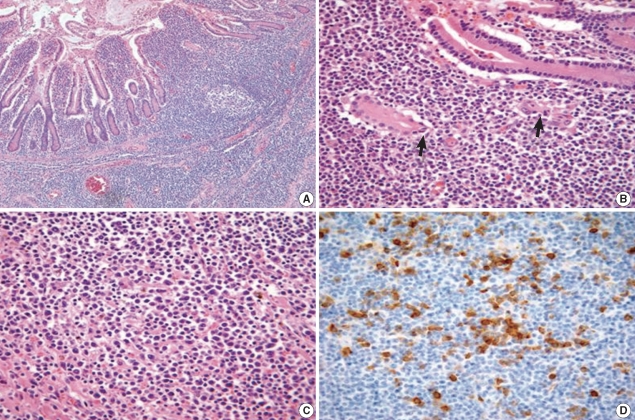
Fig. 3
Findings of peripheral blood smear and bone marrow study. (A) The peripheral blood smear shows a plasmacytoid lymphocyte (Wright stain, ×1,000). (B) The bone marrow aspirate contains 2.1% plasma cells (Wright stain, ×1,000). (C) Bone marrow biopsy showing interstitial infiltration of mature lymphocytes (hematoxylin and eosin stain, ×400).




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download