Abstract
Background
Early diagnosis is the cornerstone of management of acute myocardial infarction (AMI). We aimed to compare the diagnostic accuracy of high-sensitivity troponin T (hs-cTnT) with myeloperoxidase (MPO) and pregnancy-associated plasma protein A (PAPP-A) for early diagnosis of AMI in patients at the time of presentation to the emergency department (ED).
Methods
We enrolled 289 patients who presented at the ED of the National Institute of Heart Disease (NIHD) Rawalpindi, Pakistan, within 4 hr of onset of chest pain. Clinical assessment, electrocardiography (ECG), and angiography were carried out. Blood samples were collected at 0, 3, 6, and 12 hr. Analyses of plasma hs-cTnT, MPO, and PAPP-A were carried out using commercial kits.
Results
Out of 289 subjects who presented to the ED, we diagnosed 180 patients with coronary heart disease as having AMI (N= 61) and 119 as without AMI (stable coronary artery disease, N=61; unstable angina, N=58). Compared to non-AMI patients, the patients with AMI had significantly higher levels (represented here as median [inter quartile range]) of plasma hs-cTnT (136 [39-370] vs. 12 [7-21] ng/L), MPO (906 [564-1,631] vs. 786 [351-1,299] pmol/L) and PAPP-A (5.78 [2.67-13.4] vs. 2.8 [1.8-4.9] mIU/L). Receiver operator characteristic curves (95% CI) for hs-cTnT (0.952 [0.909-0.978]) were significantly higher (P<0.001) than those for MPO (0.886 [0.830-0.929]) and PAPP-A (0.797 [0.730-0.854]), with AMI sensitivity and specificity percentages of 87% and 98% (hs-cTnT), 82% and 84% (MPO), and 65% and 87% (PAPP-A), respectively.
Coronary heart disease (CHD) is primarily the hallmark of gradual atherosclerosis, comprising stable coronary artery disease (SCAD), unstable angina (UA), and acute myocardial infarction (AMI) due to impaired myocardial blood supply [1]. Myocardial necrosis is detected on the basis of elevated cardiac troponin levels and changes in electrocardiography (ECG) patterns in patients presenting with chest pain [1]. However, ECG and conventional cardiac biomarkers are not sensitive enough for the early diagnosis of AMI patients in the emergency department (ED) of hospitals [2]. A delay in the diagnosis of AMI increases the risk of complications and reduces the benefit of reperfusion treatment [3]. Therefore, early and specific diagnosis of AMI is the cornerstone of management of these patients in the coronary care unit and helps avoid unnecessary admissions.
According to current guidelines, patients can be diagnosed as having AMI if they have a history of chest pain, abnormal ECG changes, and a cardiac troponin value exceeding the 99th percentile in the reference population [1]. However, conventional cardiac troponin assays have low sensitivity during the initial few hours after the onset of chest pain; as a result, the diagnosis of AMI in the ED is challenging. High-sensitivity cardiac troponin T (hs-cTnT) assays with lower limits of detection have been recently introduced to overcome this problem in clinical practice. Their improved sensitivity may facilitate earlier diagnosis of AMI among patients presenting with chest pain [4].
Myeloperoxidase (MPO) is an enzyme released from neutrophils in response to acute inflammatory changes following myocardial injury. It has been suggested to be an early diagnostic marker for acute coronary syndrome [5]. Patients with AMI presenting within 2 hr of onset of chest pain have been reported to have significantly higher levels of MPO than healthy controls. It has therefore been sugges-ted that measurement of MPO may be useful for the early diagnosis of AMI [6].
Pregnancy-associated plasma protein A (PAPP-A) has been found to be a useful biomarker for predicting the rupture of unstable atherosclerotic plaques [7, 8]. However, stu-dies investigating its usefulness in the diagnosis of AMI are scant. Iversen and co-workers have recently reported that PAPP-A is a more sensitive marker for the diagnosis of AMI than troponin T and the creatine kinase isotype CK-MB [9]. Multiple cardiac biomarkers, including MPO, PAPP-A, and cardiac troponin, have been used for AMI diagnosis in the ED setting. However, the sensitivity of cardiac troponin for the diagnosis of AMI was observed to be lower within 4 hr of chest pain (55%) than at ≥12 hr (97%) after presentation at the ED [10].
The performance of new high-sensitivity cardiac biomarkers of different pathophysiological pathways needs to be evaluated for early triage and diagnosis of AMI among patients presenting with chest pain. We aimed to compare the diagnostic accuracy of newly developed hs-cTnT with that of MPO and PAPP-A assays, for the early diagnosis of AMI among CHD patients who presented at the ED of the National Institute of Heart Disease (NIHD) Rawalpindi, Pakistan, with recent onset of chest pain.
The comparative study was carried out at the Chemical Pathology Department, Army Medical College (AM College), Rawalpindi, in collaboration with NIHD, Rawalpindi, Pakistan. The research protocol was approved by the ethics committee of the AM College, National University of Science and Technology (NUST) Islamabad, Pakistan, and complies with the Declaration of Helsinki.
We enrolled 289 consecutive adult male and female pa tients aged 35 to 80 yr, who presented to the ED of NIHD within 4 hr of onset of chest pain between September 2009 and March 2010. All patients underwent an initial clinical assessment by a cardiologist, which included recording of clinical history, physical examination, 12-lead ECG, continuous monitoring, and serial hs-cTnT testing at presentation and 0, 3, 6, and 12 hr after onset of chest pain. Coronary artery angiography was carried out at NIHD.
Data were reviewed by 2 independent cardiologists for a final diagnosis at the time of discharge from the institute. We diagnosed 180 CHD patients on the basis of clinical findings, ECG, hs-cTnT, and angiography results; these included 61 patients with AMI, 58 patients with UA, and 61 patients with SCAD. AMI was diagnosed, on the basis of current guidelines, if the hs-cTnT level was at least one value above the 99th percentile with a coefficient of variation (CV) of 9.2%; if there was a >50% increase or decrease in hs-cTnT levels in 2 consecutive samples along with ECG changes were indicative of ST segment elevation/depression or development of new pathologic Q waves on serial measurements (1). UA was diagnosed in patients with chest pain lasting more than 20 min at rest, without elevation in hs-cTnT levels and with or without changes in ECG patterns. SCAD was diagnosed if angiography results revealed >70% stenosis in one of the main coronary arteries, with a history of no significant change in frequency or duration of angina on minimal exertion or at rest for at least 60 days, and if there was no evidence of recent myocardial damage. We excluded 109 patients, including those who presented with other cardiac diseases (cardiomyopathies, myocarditis, heart failure, AMI with concomitant renal failure), present-ed with pulmonary or psychiatric problems, died in the ED, or were unwilling to participate in the study.
Blood samples were collected in EDTA tubes from all patients before the administration of heparin. The samples were transported immediately to the clinical pathology laboratory of AM College, Rawalpindi, where plasma was separated by centrifugation at 2,000×g for 15 min. Assays for hs-cTnT and MPO were performed immediately after sample collection, and the remaining samples were stored at -80℃. Analysis of PAPP-A and lipid profile was carried out in batches at end of the study.
Biochemical analysis was carried out by qualified laboratory technologists according to the validated manufacturer's procedures in the clinical pathology laboratory of AM College, Rawalpindi, Pakistan. Total cholesterol, plasma glucose, and serum creatinine were measured on a Selectra E Chemistry Analyzer (Vital Scientific NV, DIERN, The Netherlands) using kits provided by Pioneer Diagnostics (Pioneer, New York, NY, USA).
Assay for hs-cTnT was performed using plasma with an electrochemiluminescence-based kit (Elecsys Cobas e 411 analyzer; Roche Diagnostics, Basel, Switzerland). The lower limit of detection for the hs-cTnT assay was 1 ng/L. The assay employs 2 monoclonal antibodies specifically directed against human cardiac troponin T. The antibodies recognize 2 epitopes (amino acid positions 125-131 and 136-147) located in the central part of the cardiac troponin T protein. The procedure comprised 2 incubations, the first of which involved the sample, a biotinylated monoclonal anti-cardiac troponin T antibody, and a monoclonal anti-cardiac troponin T antibody labelled with a ruthenium complex, which together formed a sandwich complex. The reaction mixture was aspirated into the measuring cell, where the microparticles were magnetically captured onto the surface of the electrode. Application of a voltage to the electrode then induced chemiluminescent emission, which was measured by a photomultiplier, and the values were determined using a calibration curve. The CV at 14 ng/L, i.e., 99th percentile for the assay was 9.2%.
Plasma MPO was analyzed on the ARCHITECT ci4100 immunoassay analyzer (Abbott Diagnostic, Chicago, IL, USA) by using the chemiluminescent microparticle immunoassay kit of the same manufacturer, which has a measuring range of 20-10,000 pmol/L and a CV of 4.7%. The plasma MPO levels in the healthy controls were 285 to 540 pmol/L. Serum PAPP-A levels were measured by an ultrasensitive ELISA kit (IBL International GmbH, Hamburg, Germany) using the Evolis automated ELISA processor (Bio-Rad Laboratories, Hercules, CA, USA). This kit has specifically been designed for this cardiac biomarker and has a measuring range of 0-100 mIU/L. The serum PAPP-A levels in the healthy controls were 1.74-5.91 mIU/L, and the CV of the assay was 5.1%.
Statistical analysis was performed using SPSS 16 (SPSS Inc., Chicago) and MedCalc software version 9.6.4.0. The baseline characteristics are reported as percentage for categorical variables, mean (SD) for continuous normal distributed variables, and median and interquartile range (IQR) for variables with a skewed distribution. The distribution of serum hs-cTnT, PAPP-A, and plasma MPO levels were not normal; therefore, the Mann-Whitney test was applied. The area under the receiver operator characteristic curves (AUC) of the serum levels of hs-cTnT, PAPP-A, and MPO were calculated, and the C-statistic was applied. The ROC curves from these 3 assays were compared by the method of DeLong (11). We set the cutoff value as that at which the discrimination between the cases with positive and negative diagnosis is optimal. The diagnostic accuracies of hs-cTnT, PAPP-A, and MPO were assessed on the basis of the sensitivity (SN), specificity (SP), likelihood ratio (LR), and diagnostic odds ratio (DOR) of the tests. A P value of <0.05 was considered significant.
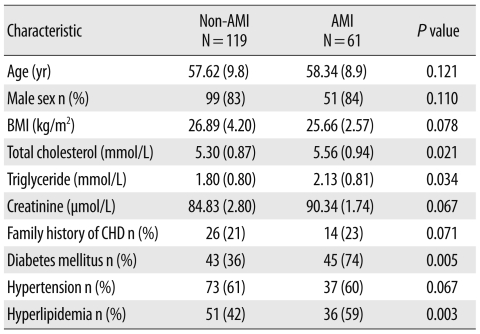
During the study period, 289 patients presented at the ED of NIHD within 4 hr of onset of chest pain. One hundred and nine patients were excluded because they had non-cardiac and cardiac diseases like cardiomyopathies, myocarditis, congestive cardiac failure, and acute coronary syndrome (ACS) with renal failure. These patients also included 4 who died in the ED and 8 who were not willing to participate in the study (Fig. 1). A total of 180 patients (age, 35 to 80 yr) with AMI (N=61) and non-AMI (N=119) diagnoses therefore constituted the final study population. The baseline characteristics of the patients are shown in Table 1. Retrosternal chest pain (98%) and sweating (61%) were the commonest presenting symptoms of patients with AMI, followed by nausea (39%), vomiting (36%), anxiety (13%), and shortness of breath (8%).
Patients with AMI had significantly higher (P<0.001) median (IQR) levels of biomarkers than non-AMI patients: hs-cTnT, 136 (39-370) ng/L vs. 12 (7-21) ng/L; MPO, 906 (564-1,631) pmol/L vs. 786 (351-1,299) pmol/L; and PAPP-A, 5.78 (2.67-13.4) mIU/L vs. 2.8 (1.8-4.9) mIU/L (Fig. 2).
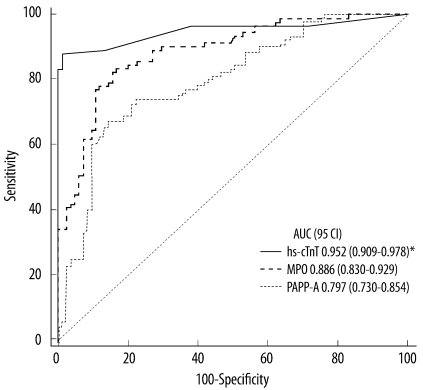
Patients with AMI had significantly higher AUC for hs-cTnT (95% CI, 0.952 [0.909-0.978]) than for MPO (0.886 [0.830-0.929]; P=0.028), or PAPP-A (0.797 [0.730-0.854]; P<0.001) at the time of presenting to the ED, as shown in Fig. 3. The AUC for MPO was also significantly higher than that for PAPP-A (P=0.015).
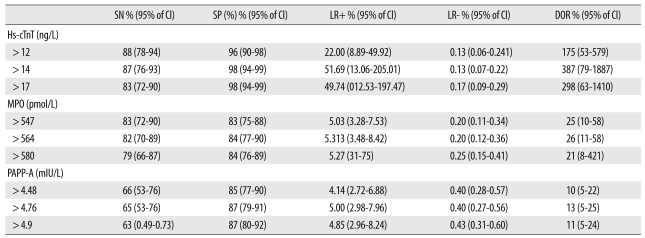
The sensitivity, specificity, positive LR, negative LR, and DOR of hs-cTnT, MPO, and PAPP-A at different cutoff levels were calculated (Table 2). The best cutoff value for hs-cTnT was 14 ng/L, with a sensitivity and specificity of 87% and 98%, respectively, for diagnosing AMI. The best cutoff values for MPO and PAPP-A were 564 pmol/L and 4.7 mIU/L respectively, with a sensitivity and specificity of 82% and 84% for MPO, and 65% and 87% for PAPP-A. On the basis of sensitivity, the number of patients with AMI correctly diagnosed at admission were 53 (87%), 50 (82%), and 39 (65%), by the hs-cTnT, MPO, and PAPP-A assays, respectively. Therefore, hs-cTnT had the highest sensitivity for the diagnosis of AMI in the ED within 4 hr of onset of chest pain.
The early triage of AMI patients who present with clinical symptoms suggestive of CHD is a challenging task for doctors. However, the development of the new high-sensitivity fifth-generation generation cardiac troponin assay, with its very low detection limits, has greatly improved the early diagnosis of minor cardiac injury. Admission of patients on provisional diagnosis of AMI without proper investigation with cardiac biomarkers, often leads to overcrowding and is associated with excessive hospital costs.
One potential advantage of a multi-marker approach is the rapid diagnosis or exclusion of AMI. cTn measurements are essential for the diagnosis of AMI in all patients who present with chest pain in the ED. Sampling of conventional cTn is necessary at presentation and at intervals of 6, 9, 12, and 24 hr. However, our main aim was to diagnose AMI patients early by using hs-cTnT; therefore, we collected samples at intervals of 0, 3, 6, and 9 hr. We also compared the diagnostic usefulness of newer-generation hs-cTnT with MPO and PAPP-A assays for the early detection of AMI in patients of CHD presenting with chest pain. The AUC values showed that the hs-cTnT assay has excellent diagnostic ability for patients presenting at the ED within 4 hr of onset of chest pain. The number of patients correctly classified at 4 hr was also the highest when plasma hs-cTnT levels were used. This demonstrates that the diagnostic performance of hs-cTnT is superior to that of the MPO and PAPP-A assays.
The AUC values for hs-cTnT noted in our AMI patients at the time of admission to ED were similar to those reported by Reichlin et al. [4]. The sensitivity and specificity of the hs-cTnT assay was excellent for early diagnosis of AMI, with a cutoff value greater than 14 ng/L at the 99th percentile. Keller et al. [12] also reported a higher AUC value with a high-sensitivity troponin I assay than with the conventional troponin T assay Standard troponin assays have several limitations. When measured 4 hr after the onset of chest pain, their sensitivity and specificity are 35% and 96% respectively [13]. Further, they are incapable of determining concentrations in a healthy population. Another study demonstrated that use of the 99th percentile cutoff for the hs-cTnT assay allows earlier diagnosis of non-ST-elevation myocardial infarction (NSTEMI) [14]. Our study supports the previous finding that cardiac troponin is more clinically accurate for the early diagnosis of AMI [15]. An ideal biochemical marker would therefore be one that has high sensitivity and specificity and appears early after an AMI.
The levels of MPO observed in our study were similar to those observed by Khan et al. [16] and Mocatta et al. [17]. Ndrepepa et al. [18] also found higher circulating levels of MPO in patients of AMI than in patients with angina and healthy individuals. Elevated levels of PAPP-A in AMI patients were previously reported by Iversen et al. [9]. Our findings differ from those of Brennan et al. with respect to the usefulness of MPO in the diagnosis of ACS in patients who were consistently negative for Troponin T [6]. They used the stan-dard troponin T assay, which has reduced sensitivity at low concentrations. However, the results of our study are consistent with the observations of Esporcatte et al. [19], who reported a diagnostic sensitivity of 92% and specificity of 40% for identifying AMI patients with acute chest pain. Elesber et al. also assessed the levels of PAPP-A in patients presenting to the ED with acute chest pain suggestive of ACS, and reported that PAPP-A levels were predictive of a final diagnosis of ACS [20]. Evidence shows that myocardial injury not only is related to platelet activation but also involves leukocyte accumulation and activation in the atherosclerotic plaque. These leukocytes undergo degranulation in the coronary circulation in patients with ACS, which leads to the release of MPO and thus accounts for the elevated levels of MPO seen in ACS. More recently, PAPP-A was assessed as a marker of vulnerable plaque in patients with ACS, and the number of patients with high-risk ACS (63%) reported to have detectable PAPP-A was significantly greater than that of patients with low-risk ACS (39%; P<0.001). We observed a strong and positive correlation between MPO and PAPP-A, which reflects a heightened state of inflammation in ACS. However, the diagnostic performance of the hs-cTnT assay used in our study was significantly higher than that of both MPO and PAPP-A assays. Research is underway to identify a biomarker that can detect ACS before the manifestation of full-blown AMI (e.g., MPO and PAPP-A), but none have shown potential for use in standard practice for diagnosis of AMI because of deficiencies in their diagnostic performance. Merits of this study is that we determined the cutoff values, sensitivity, specificity, and DOR of biomarkers by using newly validated hs-cTnT assays for early diagnosis of AMI in patients with CHD in the ED. Our study supports the recommendation for the use of hs-cTnT as a single cardiac biomarker along with ECG for early diagnosis of AMI in the emergency setup. We acknowledge the potential limitations of our study, namely, a relatively small sample size and lack of evaluation in a typical clinical setting because of our strict inclusion criteria of CHD patients only.
We conclude that high-sensitivity troponin enables a more rapid and accurate diagnosis of myocardial injury than conventional cardiac biomarkers. The diagnostic performance of hs-cTnT is superior to MPO and PAPP-A for early triage of AMI among CHD patients presenting with chest pain in an emergency medical care setting.
References
1. Thygesen K, Alpert JS, White HD. Universal definition of myocardial infarction. J Am Coll Cardiol. 2007; 50:2173–2195. PMID: 18036459.
2. Menown IB, Mackenzie G, Adgey AA. Optimizing the initial 12-lead electrocardiographic diagnosis of acute myocardial infarction. Eur Heart J. 2000; 21:275–283. PMID: 10653675.

3. Bassand JP, Hamm CW, Ardissino D, Boersma E, Budaj A, Fernández-Aviléz F, et al. Guidelines for the diagnosis and treatment of non-ST-segment elevation acute coronary syndromes. Eur Heart J. 2007; 28:1598–1660. PMID: 17569677.
4. Reichlin T, Hochholzer W, Bassetti S, Steuer S, Stelzig C, Hartwiger S, et al. Early diagnosis of myocardial infarction with sensitive cardiac troponin assays. N Engl J Med. 2009; 361:858–867. PMID: 19710484.

5. Gururajan P, Gurumurthy P, Nayar P, Babu S, Sarasabharati A, Victor D, et al. Serum myeloperoxidase: a novel biomarker for evaluation of patients with acute coronary syndrome. Heart Asia. 2009; 1:41–46.
6. Brennan ML, Penn MS, Van Lente F, Nambi V, Shishehbor MH, Aviles RJ, et al. Prognostic value of myeloperoxidase in patients with chest pain. N Engl J Med. 2003; 349:1595–1604. PMID: 14573731.

7. Bayes-Genis A, Conover CA, Overgaard MT, Bailey KR, Christiansen M, Holmes DR Jr, et al. Pregnancy-associated plasma protein A as a marker of acute coronary syndromes. N Engl J Med. 2001; 345:1022–1029. PMID: 11586954.

8. Lund J, Qin QP, Ilva T, Pettersson K, Viopio-Pulkki LM, Porela P, et al. Circulating pregnancy-associated plasma protein-A predicts outcome in patients with acute coronary syndrome but no Troponin-I elevation. Circulation. 2003; 108:1924–1926. PMID: 14530192.
9. Iversen KK, Teisner AS, Teisner B, Kliem A, Thanning P, Grande P, et al. Pregnancy associated plasma protein A, a novel, quick, and sensitive marker in ST-elevation myocardial infarction. Am J Cardiol. 2008; 101:1389–1394. PMID: 18471447.

10. McCann CJ, Glover BM, Menown IB, Moore MJ, McEneny J, Owens CG, et al. Novel biomarkers in early diagnosis of acute myocardial infarction compared with cardiac troponin T. Eur Heart J. 2008; 29:2843–2850. PMID: 18682444.

11. DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. 1988; 44:837–845. PMID: 3203132.

12. Keller T, Zeller T, Peetz D, Tzikas S, Roth A, Czyz E, et al. Sensitive troponin I assay in early diagnosis of acute myocardial infarction. N Engl J Med. 2009; 361:868–877. PMID: 19710485.

13. Achar AS, Kundu S, Norcross WA. Diagnosis of acute coronary syndrome. Am Fam Physician. 2005; 72:119–126. PMID: 16035692.
14. Giannitsis E, Becker M, Kurz K, Hess G, Zdunek D, Katus HA. High-sensitive cardiac troponin T for early prediction of evolving non-ST-segment elevation myocardial infarction in patients with suspected acute coronary syndrome and negative troponin results on admission. Clin Chem. 2010; 56:642–650. PMID: 20167697.
15. Apple FS, Smith SW, Pearce LA, Murakami MM. Assessment of the multiple-biomarker approach for diagnosis of myocardial infarction in patients presenting with symptoms suggestive of acute coronary syndrome. Clin Chem. 2009; 55:93–100. PMID: 19028826.

16. Khan SQ, Kelly D, Quinn P, Davies JE, Ng LL. Myeloperoxidase aids prognostication together with N-terminal pro-B-type natriuretic peptide in high-risk patients with acute ST elevation myocardial infarction. Heart. 2007; 93:826–831. PMID: 17194712.

17. Mocatta TJ, Pilbrow AP, Cameron VA, Senthilmohan R, Frampton CM, Richards AM, et al. Plasma concentrations of myeloperoxidase predict mortality after myocardial infarction. J Am Coll Cardiol. 2007; 49:1993–2000. PMID: 17512353.

18. Ndrepepa G, Braun S, Mehilli J, von Beckerath N, Schomig A, Kastrati A. Myeloperoxidase level in patients with stable coronary artery disease and acute coronary syndrome. Eur J Clin Invest. 2008; 38:90–96. PMID: 18226042.
19. Esporcatte R, Rey HCV, Rocha RM, Bittencourt MI, Salgado CS, Garcia MI, et al. Impact of myeloperoxidase dosage in acute coronary syndrome. Crit Care. 2005; 9(S2):S30.
20. Elesber AA, Lerman A, Denktas AE, Resch ZT, Jared Bunch T, Schwartz RS, et al. Pregnancy associated plasma protein-A and risk stratification of patients presenting with chest pain in the emergency department. Int J Cardiol. 2007; 117:365–369. PMID: 16859783.

Fig. 1
Schematic diagram showing the methodology for classification of patients.
Abbreviations: AMI, acute myocardial infarction; CAD, coronary artery disease; UA, unstable angina; SCAD, stable coronary artery disease; hs-cTnT, high-sensitivity cardiac troponin T; MPO, Myeloperoxidase; PAPP-A pregnancy-associated plasma protein A.
Fig. 2
Comparison of high-sensitivity cardiac troponin, myeloperoxidase and pregnancy-associated plasma protein A levels between patients with AMI (N=61) and without AMI at admission (N=119).
Abbreviations: AMI, acute myocardial infarction; MPO, Myeloperoxidase; PAPP-A, pregnancy-associated plasma protein A.
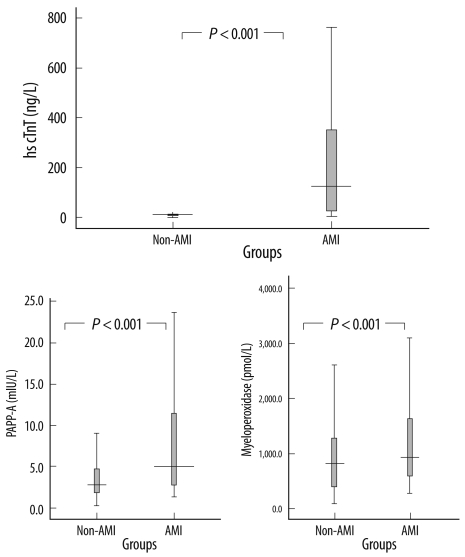
Fig. 3
Comparison of area under receiver operator characteristic curves for high-sensitivity cardiac troponin (hs-cTnT), myeloperoxidase (MPO), and pregnancy-associated plasma protein A (PAPP-A) between patients with and without AMI at presentation in the ED. P value of hs-cTnT is <0.01 when compared to MPO and PAPP-A.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download