1. Zhang Y, Poetsch M, Weber-Matthiesen K, Rohde K, Winkemann M, Haferlach T, et al. Secondary acute leukaemias with 11q23 rearrangement: clinical, cytogenetic, FISH and FICTION studies. Br J Haematol. 1996; 92:673–680. PMID:
8616034.

2. Ishizawa S, Slovak ML, Popplewell L, Bedell V, Wrede JE, Carter NH, et al. High frequency of pro-B acute lymphoblastic leukemia in adults with secondary leukemia with 11q23 abnormalities. Leukemia. 2003; 17:1091–1095. PMID:
12764373.

3. Pagano L, Pulsoni A, Mele L, Leone G. Clinical and epidemiological features of acute lymphoblastic leukemia following a previous malignancy. Leuk Lymphoma. 2000; 39:465–475. PMID:
11342330.

4. Secker-Walker LM, Moorman AV, Bain BJ, Mehta AB. Secondary acute leukemia and myelodysplastic syndrome with 11q23 abnormalities. EU Concerted Action 11q23 Workshop. Leukemia. 1998; 12:840–844. PMID:
9593290.
5. Chen W, Wang E, Lu Y, Gaal KK, Huang Q. Therapy-related acute lymphoblastic leukemia without 11q23 abnormality: report of six cases and a literature review. Am J Clin Pathol. 2010; 133:75–82. PMID:
20023261.
6. Hrusák O, Porwit-MacDonald A. Antigen expression patterns reflecting genotype of acute leukemias. Leukemia. 2002; 16:1233–1258. PMID:
12094248.

7. Andersen MK, Christiansen DH, Jensen BA, Ernst P, Hauge G, Pedersen-Bjergaard J. Therapy-related acute lymphoblastic leukaemia with MLL rearrangements following DNA topoisomerase II inhibitors, an increasing problem: report on two new cases and review of the literature since 1992. Br J Haematol. 2001; 114:539–543. PMID:
11552977.

8. Cho J, Hur M, Moon HW, Yun YM, Lee CH, Lee HG. A case of therapy-related ALL with MLL gene rearrangement following treatment of breast cancer. Korean J Lab Med. 2010; 30:255–259. PMID:
20603585.

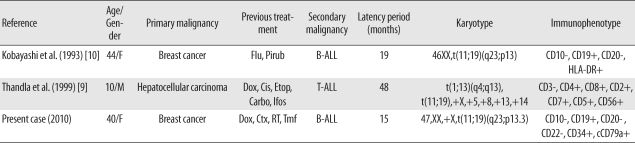
9. Thandla S, Alashari M, Green DM, Aplan PD. Therapy-related T cell lymphoblastic lymphoma with t(11;19)(q23;p13) and MLL gene rearrangement. Leukemia. 1999; 13:2116–2118. PMID:
10602439.

10. Kobayashi Y, Yang J, Shindo E, Tojo A, Tani K, Ozawa K, et al. HRX gene rearrangement in acute lymphoblastic leukemia after adjuvant chemotherapy of breast cancer. Blood. 1993; 82:3220–3221. PMID:
8219210.
11. Burmeister T, Meyer C, Schwartz S, Hofmann J, Molkentin M, Kowarz E, et al. The MLL recombinome of adult CD10-negative B-cell precursor acute lymphoblastic leukemia: results from the GMALL study group. Blood. 2009; 113:4011–4015. PMID:
19144982.

12. Huret JL, Brizard A, Slater R, Charrin C, Bertheas MF, Guilhot F, et al. Cytogenetic heterogeneity in t(11;19) acute leukemia: clinical, hematological and cytogenetic analyses of 48 patients--updated published cases and 16 new observations. Leukemia. 1993; 7:152–160. PMID:
8426468.
13. Mitani K, Sato Y, Kobayashi Y, Shibasaki Y, Kasuga M, Inaba T, et al. Heterogeneity in the breakpoints of chromosome 19 among acute leukemic patients with the t(11;19)(q23;p13) translocation. Am J Hematol. 1989; 31:253–257. PMID:
2741924.
14. Hudson MM, Raimondi SC, Behm FG, Pui CH. Childhood acute leukemia with t(11;19) (q23;p13). Leukemia. 1991; 5:1064–1068. PMID:
1774955.
15. Moorman AV, Hagemeijer A, Charrin C, Rieder H, Secker-Walker LM. European 11q23 Workshop participants. The translocations, t(11;19)(q23;p13.1) and t(11;19)(q23;p13.3): a cytogenetic and clinical profile of 53 patients. Leukemia. 1998; 12:805–810. PMID:
9593285.

16. Fu JF, Liang DC, Shih LY. Analysis of acute leukemias with MLL/ENL fusion transcripts: identification of two novel breakpoints in ENL. Am J Clin Pathol. 2007; 127:24–30. PMID:
17145626.
17. Rubnitz JE, Camitta BM, Mahmoud H, Raimondi SC, Carroll AJ, Borowitz MJ, et al. Childhood acute lymphoblastic leukemia with the MLL-ENL fusion and t(11;19)(q23;p13.3) translocation. J Clin Oncol. 1999; 17:191–196. PMID:
10458233.

18. Pui CH, Behm FG, Downing JR, Hancock ML, Shurtleff SA, Ribeiro RC, et al. 11q23/MLL rearrangement confers a poor prognosis in infants with acute lymphoblastic leukemia. J Clin Oncol. 1994; 12:909–915. PMID:
8164041.

19. Rubnitz JE, Link MP, Shuster JJ, Carroll AJ, Hakami N, Frankel LS, et al. Frequency and prognostic significance of HRX rearrangements in infant acute lymphoblastic leukemia: a Pediatric Oncology Group study. Blood. 1994; 84:570–573. PMID:
8025282.

20. Schoch C, Schnittger S, Klaus M, Kern W, Hiddemann W, Haferlach T. AML with 11q23/MLL abnormalities as defined by the WHO classification: incidence, partner chromosomes, FAB subtype, age distribution, and prognostic impact in an unselected series of 1897 cytogenetically analyzed AML cases. Blood. 2003; 102:2395–2402. PMID:
12805060.

21. Pui CH, Chessells JM, Camitta B, Baruchel A, Biondi A, Boyett JM, et al. Clinical heterogeneity in childhood acute lymphoblastic leukemia with 11q23 rearrangements. Leukemia. 2003; 17:700–706. PMID:
12682627.

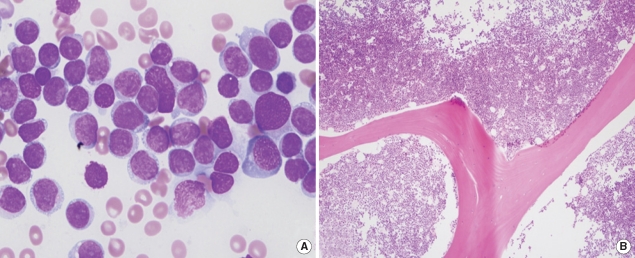
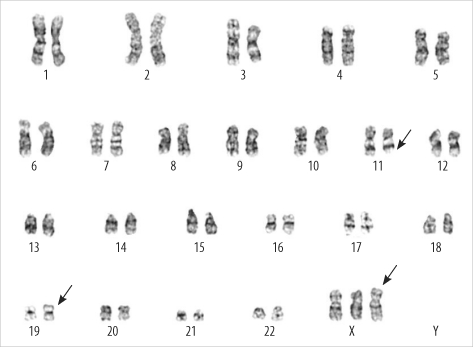
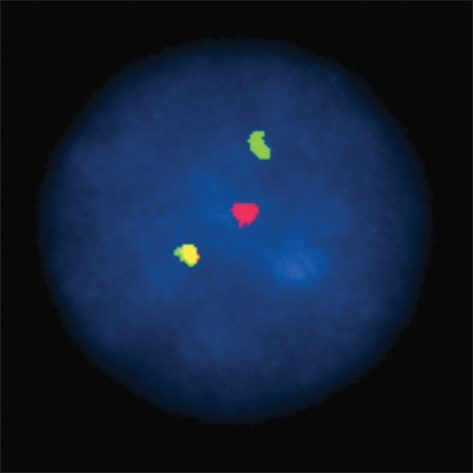
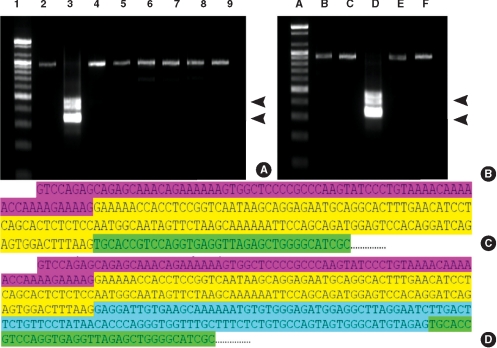




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download