Abstract
Propionibacterium acnes is a gram-positive anaerobic bacillus and a normal inhabitant of the skin. Although it is often considered a contaminant of blood cultures, it can occasionally cause serious infections, including postoperative central nervous system infections. Here, we report the case of a 70-yr-old man who developed a large cerebral abscess caused by P. acnes 13 months after neurosurgery. Immediate gram staining of the pus from his brain revealed the presence of gram-positive coccobacilli. However, colony growth was observed only after 5 days of culture. Therefore, we performed 16S rRNA gene sequencing of the pus specimen. The isolate was identified as P. acnes. The colonies developed 9 days after the initial culture. The API Rapid ID 32A test (bioMérieux, France) was performed using a colony, but an unacceptable profile was obtained. Then, the pus was transferred into the enrichment broths of the BACTEC FX (Becton Dickinson, USA) and BacT/Alert 3D (bioMérieux, Organon Teknika, USA) systems, but only the BACTEC FX system could detect growth after 5 days. We performed 16S rRNA gene sequencing and API Rapid 32A profiling with a colony recovered from Brucella agar, which was inoculated with the microbial growth in the enrichment broth from the BACTEC FX system. The organism was identified as P. acnes by both methods. This case suggests that 16S rRNA gene sequencing may be a useful alternative for identifying slowly growing P. acnes from specimens that do not show growth after 5 days of culture.
Propionibacterium acnes is a gram-positive, slow-growing, anaerobic bacillus and a part of the normal flora of the skin, nasopharynx, oral cavity, and gastrointestinal and genitourinary tracts [1]. Although P. acnes is a frequent contaminant of the cultures obtained percutaneously, it has been implicated as the cause of central nervous system (CNS) infections such as brain abscesses and subdural and epidural empyemas [2]. Most reported cases of P. acnes CNS infections occur in association with shunt infections, and these infections are rarely found in the absence of infected hardware [3]. In Korea, one study reported a case of P. acnes CNS infection in a patient with chronic subdural hematoma who presented with subdural empyema and had not undergone neurosurgery [4]. The identification of P. acnes as the causative organism may be difficult because of its slow growth, which makes rapid and accurate identification an important consideration in differential diagnosis. Here, we describe a case of large cerebral abscess caused by P. acnes 13 months after neurosurgery and confirmed by 16S rRNA gene sequencing.
A 70-yr-old man with pus discharge from a postoperative wound was admitted to the Seoul National University Hospital. The patient had undergone decompressive craniectomy and partial lobectomy 13 months before admission because of cerebral infarction and subsequent brain swelling. During rehabilitation, at 10 months after the neurosurgery, the postoperative wound started to bulge. Three days before admission, the patient showed pus discharge from the wound and started to experience pain. Initial vital signs were as follows: blood pressure, 128/84 mmHg; pulse rate, 50/min; respiratory rate, 18/min; and body temperature, 36.0℃. Initial laboratory test results were as follows: Hb, 12.9 g/dL; white blood cell count, 7.3×109/L (neutrophils, 53%; lymphocytes, 34%; monocytes, 9%; eosinophils, 3%; and basophils, 1%); platelets, 189×109/L; and C-reactive protein level, 0.17 mg/dL. Results of the liver function tests and creatinine and glucose levels were within reference ranges. Brain magnetic resonance imaging revealed fluid collection, with peripheral enhancement in the frontotemporal area and diffuse restriction suggestive of brain abscess. The patient underwent urgent surgery via craniotomy. After removal of the bone flap, yellowish pus was expelled, and large amounts of purulent materials were found at the subdural area. The pus was transferred into several conical tubes and sent to a microbiology laboratory. Immediate gram staining revealed gram-positive coccobacilli (Fig. 1A). The pus was cultured under anaerobic conditions (80:10:10 atmosphere of N2:H2:CO2 at 37℃) by using the MACS MG500 Anaerobic Workstation (Don Whitley Scientific, West Yorkshire, UK). However, no bacterial growth was observed after 5 days of culture. Therefore, we performed 16S rRNA gene sequencing with the initial pus specimen by using MicroSeq 500 16S rRNA Bacterial Identification Kits (PN 4346298; Applied Biosystems, Foster City, CA, USA), according to the manufacturer's instructions. The sequence was analyzed using the MicroSeq ID 16S rRNA 500 Library v2.1 and analysis software v2.0 (Applied Biosystems). The isolate was identified as P. acnes (100.00% match) according to the identity algorithm of the CLSI guideline MM18-A on gram-positive anaerobes [5]. Nine days after the culture of the initial pus, circular, convex, and opaque colonies with a diameter of 1-2 mm were observed on Brucella agar, and the organisms were found to be gram-positive rods (Fig. 1B). The API Rapid ID 32A test (bioMérieux, Marcy-l'Etoile, France) was performed using a colony; however, the result revealed an unacceptable profile for P. acnes (Profile 2103771604) (Table 1). It indicated positive activity of alkaline phosphatase and phenylalanine arylamidase, which are not usually observed in P. acnes. In addition, the pus specimen was transferred to the enrichment broths of the BACTEC FX (Becton Dickinson, Sparks, MD, USA) and BacT/Alert (bioMérieux, Organon Teknika, Durham, NC, USA) systems, and growth was obtained only in the BACTEC FX system after incubation for 5 days. The enrichment broth from the BACTEC system was inoculated on Brucella agar. After 2 days of incubation under anaerobic conditions, colonies developed on Brucella agar. 16S rRNA gene sequencing was then performed using a colony from the Brucella agar. The isolate was identified as P. acnes (99.77% match). API Rapid ID 32A test was also performed using a colony from Brucella agar, and the organism was identified as P. acnes (Profile 2103770604, % ID: 99.9, T index: 0.43). At this time, phenylalanine arylamidase activity was found to be absent. Therefore, P. acnes was thought to be the causative organism for the purulent pus and large brain abscess. The organism was found to be β-lactamase negative. The patient received intravenous vancomycin and ceftazidime treatment for 2 weeks and was then discharged.
P. acnes is likely to cause infection after intracranial operative procedures because of its prevalence in the scalp flora, where it resides in the anaerobic environment of the hair follicle [6]. In a retrospective review of the postoperative courses of 2,111 patients who underwent neurosurgical procedures, the incidence of postoperative CNS infections (PCNSIs) caused by P. acnes (4 cases; 25% of the infections) was second only to that of infections caused by Staphylococcus aureus (8 cases; 50% of the infections) [7]. Although, in the above-mentioned study, the PCNSIs caused by P. acnes were associated with ventriculoperitoneal shunts and Ommaya reservoir placement, cases involving intracranial operations have been well described in other studies [2, 6, 8, 9].
P. acnes is often not readily identified as a cause of PCNSI because the signs of infection are observed only after a delay and because P. acnes has a slow-growing nature. Our finding of brain abscess caused by P. acnes, which appeared 13 months after neurosurgery, was consistent with previous findings (21 days to 10 yr). Furthermore, colonial growth was first observed 9 days after inoculating the pus on Brucella agar, as expected from previous reports (2-9 days, median: 4 days) [8].
The results of the API rapid ID 32A test performed using the colonies obtained after direct pus inoculation and those from Brucella agar, which was inoculated with the microbial growth in the BACTEC enrichment broth, were somewhat discrepant. This can be explained on the basis of previous findings. In a previous study on the exoenzymes of P. acnes, it was observed that the activity of many of the enzymes of P. acnes appeared to be dependent on the culture substrate [10]. In that study, the researchers used the API ZYM System (Analytab Products Inc., Plainview, NY, USA), which is the predecessor of the ATB 32A System (now renamed as Rapid ID 32A). Regarding the exoenzymes of the 30 strains of P. acnes grown in 6 different media, including 5% sheep blood agar, the percentages of the strains exhibiting strong enzymatic activity varied depending on the type of medium. For example, 80% of the tested P. acnes strains exhibited α-galactosidase activity in thioglycollate medium, but only 10% of the strains exhibited α-galactosidase activity in 5% sheep blood agar. Moreover, a study evaluating the use of the ATB 32A system for identifying anaerobic bacteria isolated from clinical specimens supported this finding [11]. This study, which was performed using colonies grown on Columbia sheep blood agar, revealed that some strains of anaerobic bacteria, including Propionibacterium spp., showed false-positive or false-negative results for various enzymes, leading to unacceptable profiles or misidentification of the species. One of the 11 tested P. acnes, ATCC 6919, showed false-positive α-glucosidase activity, weak β-N-acetyl-glucosaminidase activity, and a false-negative nitrate reduction test result, thereby presenting an unacceptable profile. In this study, the separation index of 260 strains of anaerobic bacteria was calculated. The result showed that the separation index of alkaline phosphatase was 2-fold higher than that of phenylalanine arylamidase (1,440 vs. 704), indicating that the discriminatory power of alkaline phosphatase for the identification P. acnes is higher than that of phenylalanine arylamidase. However, in our case, the ability to identify P. acnes was not dependent on the negative results of alkaline phosphatase activity; in fact, the negative results of phenylalanine arylamidase activity were important for profiling P. acnes. We assume that this is because the colony we used in the API test was selected from those growing on Brucella agar, and the substrate in the Brucella agar might have affected the results of the enzyme activity analysis.
After transferring the pus into the enrichment broth of both BACTEC and BacT/Alert systems, we observed growth only in the BACTEC system after 5 days of incubation. The time-to-positivity (TTP) of P. acnes was studied using only the BACTEC 9240 system (56.8±19.5 hr), and there were no comparative data of the TTP of the 2 systems [12]. Earlier, studies on identifying the recovered date were performed using both systems. In a previous study, the BACTEC 9240 system was used to study 80 culture specimens recovered as Propionibacterium spp., out of which 48 (60.0%) were identified as positive for Propionibacterium spp. after 120 hr, 66 (82.5%) were identified as positive after 144 hr, and 80 (100.0%) were identified as positive after 192 hr [13]. However, in another study, when the BacT/Alert system was used to evaluate 198 positive cultures, the cumulative percentage of the positive cultures was 23% after 120 hr, 63% after 144 hr, and 100% after 168 hr [14]. Therefore, with an incubation time of 5 days, the Propionibacterium spp. would go undetected in 77% of the positive samples if the BacT/Alert system was used and in 40% of the positive samples if the BACTEC 9240 system was used.
The reason for the prolonged detection time in the BacT/Alert system, in comparison to that in the BACTEC system, has not been explained. Several hypotheses can be suggested to explain this. The factors that might affect TTP are the composition of the media, the texture of the culture bottle, and the detection system itself. However, the enrichment broths of both systems are not significantly different and contain soybean-casein digest with vitamins, amino acids, and carbohydrates. Further, although the culture bottles in the BACTEC system and the BacT/Alert system were made of glass and plastic, respectively, a recent study concluded that the performance of the newly invented plastic bottles was similar to that of the glass bottles used in the BacT/Alert system [15]. Therefore, the difference in detection times is possibly because of the computer algorithm modifications in each system. In our case, we did not attempt to detect P. acnes using the BacT/Alert system after 7 days of incubation. However, we believe that if the incubation had been carried out for a longer period, the BacT/Alert system may have detected the organism.
PCR amplification and sequencing of the 16S rRNA gene have been used successfully in previous studies to detect the slow-growing P. acnes in vascular prosthesis infections and infective heart valve endocarditis [16, 17]. In these cases, no bacteria were observed in the Gram stain, and no growth was observed after culturing. Furthermore, a recent study revealed that 16S rRNA gene sequencing performed simultaneously with the conventional identification methods of detecting anaerobic bacteria from blood cultures offered benefits over the conventional methods of identification [18]. They reported improvement in the percentage of identification (up to the species level) and in the detection time. This combined method might be superior to culture methods in the diagnosis of bacterial infections if the patient is receiving empirical antibiotics, if the infection is caused by bacteria with unusual growth requirements, or if the pathogen is of an unexpected strain [16].
Although we could not determine the minimum inhibitory concentration, P. acnes is susceptible to many antibiotics, including the majority of the β-lactams, clindamycin, vancomycin, and fluoroquinolones [8]. The P. acnes isolate obtained from our patient was β-lactamase negative, and the infection was resolved after administering vancomycin and ceftazidime.
In summary, using conventional methods with the API rapid ID 32A kit or enrichment broths, and especially using only the BacT/Alert system, could result in misidentification, no identification, or delayed identification of P. acnes. In our case, had we not performed 16S rRNA gene sequencing, the detection time, workload, and the costs of identifying the correct pathogen would have increased. Therefore, 16S rRNA gene sequencing could be a useful alternative method for the rapid and timely detection of P. acnes in PCNSIs.
References
1. Brook I, Frazier EH. Infections caused by Propionibacterium species. Rev Infect Dis. 1991; 13:819–822. PMID: 1962090.
2. Ramos JM, Esteban J, Soriano F. Isolation of Propionibacterium acnes from central nervous system infections. Anaerobe. 1995; 1:17–20. PMID: 16887502.
3. Conen A, Walti LN, Merlo A, Fluckiger U, Battegay M, Trampuz A. Characteristics and treatment outcome of cerebrospinal fluid shunt-associated infections in adults: a retrospective analysis over an 11-year period. Clin Infect Dis. 2008; 47:73–82. PMID: 18484878.

4. Kim JH, Lee CH, Hwang SH, Kang DH. Superimposed Propionibacterium acnes subdural empyema in a patient with chronic subdural hematoma. J Korean Neurosurg Soc. 2009; 45:53–56. PMID: 19242574.
5. Clinical and Laboratory Standards Institute. Interpretive criteria for identification of bacteria and fungi by DNA target sequencing: approved guideline. MM18-A. 2008. Wayne; PA: Clinical and Laboratory Standards Institute.
6. Kranick SM, Vinnard C, Kolson DL. Propionibacterium acnes brain abscess appearing 10 years after neurosurgery. Arch Neurol. 2009; 66:793–795. PMID: 19506144.

7. McClelland S 3rd, Hall WA. Postoperative central nervous system infection: incidence and associated factors in 2111 neurosurgical procedures. Clin Infect Dis. 2007; 45:55–59. PMID: 17554701.

8. Nisbet M, Briggs S, Ellis-Pegler R, Thomas M, Holland D. Propionibacterium acnes: an under-appreciated cause of post-neurosurgical infection. J Antimicrob Chemother. 2007; 60:1097–1103. PMID: 17875606.
9. Barazi SA, Gnanalingham KK, Chopra I, van Dellen JR. Delayed postoperative intracerebral abscess caused by Proprionibacterium acnes: case report and review of the literature. Br J Neurosurg. 2003; 17:336–339. PMID: 14579899.
10. Kabongo Muamba ML. Exoenzymes of Propionibacterium acnes. Can J Microbiol. 1982; 28:758–761. PMID: 7172137.
11. Looney WJ, Gallusser AJ, Modde HK. Evaluation of the ATB 32 A system for identification of anaerobic bacteria isolated from clinical specimens. J Clin Microbiol. 1990; 28:1519–1524. PMID: 2199516.

12. Park SH, Shim H, Yoon NS, Kim MN. Clinical relevance of time-to-positivity in BACTEC9240 blood culture system. Korean J Lab Med. 2010; 30:276–283. PMID: 20603588.

13. Reisner BS, Woods GL. Times to detection of bacteria and yea-sts in BACTEC 9240 blood culture bottles. J Clin Microbiol. 1999; 37:2024–2026. PMID: 10325369.

14. Hardy DJ, Hulbert BB, Migneault PC. Time to detection of positive BacT/Alert blood cultures and lack of need for routine subculture of 5- to 7-day negative cultures. J Clin Microbiol. 1992; 30:2743–2745. PMID: 1400979.

15. Mirrett S, Joyce MJ, Reller LB. Validation of performance of plastic versus glass bottles for culturing anaerobes from blood in BacT/ALERT SN medium. J Clin Microbiol. 2005; 43:6150–6151. PMID: 16333117.

16. Le Page L, Podglajen I, Chemla E, Mainardi JL. Molecular diagnosis of a vascular prosthesis infection, due to Propionibacterium acnes, by amplification and sequencing of 16S rDNA. Clin Microbiol Infect. 2003; 9:1125–1127. PMID: 14616731.
17. Breitkopf C, Hammel D, Scheld HH, Peters G, Becker K. Impact of a molecular approach to improve the microbiological diagnosis of infective heart valve endocarditis. Circulation. 2005; 111:1415–1421. PMID: 15753218.

18. Justesen US, Skov MN, Knudsen E, Holt HM, Søgaard P, Justesen T. 16S rRNA gene sequencing in routine identification of anaerobic bacteria isolated from blood cultures. J Clin Microbiol. 2010; 48:946–948. PMID: 20071555.

Fig. 1
(A) Immediate gram staining of the pus showing gram-positive coccobacilli (×1,000); (B) Gram staining of a colony from the pus cultured anaerobically on Brucella agar showing gram-positive bacilli (×1,000).

Table 1
The API rapid ID 32A test (bioMérieux) results for the colonies obtained after direct pus inoculation and those obtained from Brucella agar, which was inoculated with the microbial growth in the BACTEC enrichment broth (Becton Dickinson)
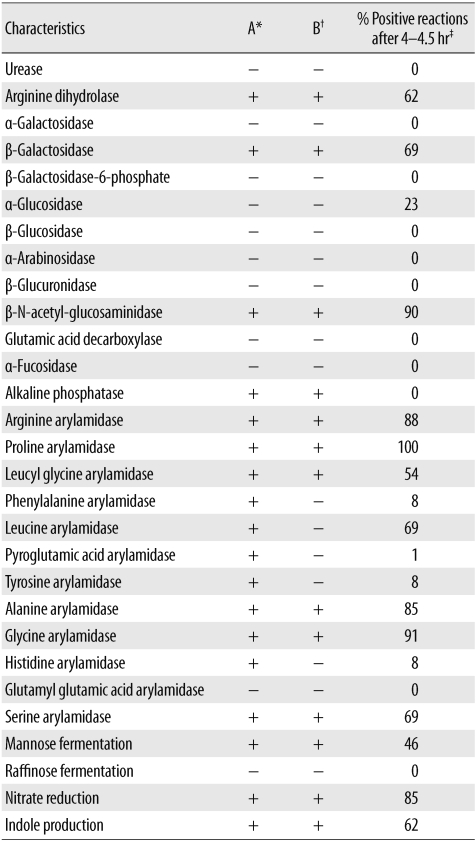
*API rapid ID 32A test (bioMérieux) results for the colonies obtained after direct pus inoculation, which was inoculated with the microbial growth in the BACTEC enrichment broth (Becton Dickinson); †API rapid ID 32A test results for the colonies obtained after direct pus inoculation, which was inoculated with the microbial growth in the BACTEC enrichment broth; ‡Data from the insert sheet of the API rapid ID 32A kit.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download