Abstract
Background:
Malignant hyperthermia (MH) is genetically heterogeneous, with mutations in the gene encoding the skeletal muscle ryanodine receptor (RYR1) at 19q13.1 accounting for up to 80% of the cases. However, the search for known and novel mutations in the RYR1 gene is hampered by the fact that the gene contains 106 exons. We aimed to analyze mutations from the entire RYR1 coding region in Korean MH families.
Methods:
We investigated seven affected MH individuals and their family members. The entire RYR1 coding region from the genomic DNA was sequenced, and RYR1 haplotyping and mutational analysis were carried out.
Results:
We identified nine different RYR1 mutations or variations from seven Korean MH families. Among these, five previously reported mutations (p.Gly248Arg, p.Arg2435His, p.Arg2458His, p.Arg-2676Trp, and p.Leu4838Val) and four novel variations of unknown significance (p.Arg2508Cys, p.Met-4022Val, p.Glu2669Lys, and p.Ala4295Val) were identified. In two families, two variations (R2676W & M4022V, R2435H & A4295V, respectively) were identified simultaneously. Four of the observed nine mutations or variations were located outside the hotspot region of RYR1 mutations.
Conclusions:
These data indicate that RYR1 is a main candidate gene in Korean MH families, and that comprehensive screening of the entire coding sequence of the RYR1 gene is necessary for molecular genetic investigations in MH-susceptible individuals, owing to the presence of RYR1 mutations or variations outside of the hotspot region.
REFERENCES
1.Jurkat-Rott K., McCarthy T., Lehmann-Horn F. Genetics and pathogenesis of malignant hyperthermia. Muscle Nerve. 2000. 23:4–17.

2.López JR., Allen PD., Alamo L., Jones D., Sreter FA. Myoplasmic free [Ca2+] during a malignant hyperthermia episode in swine. Muscle Nerve. 1988. 11:82–8.

3.Allen GC., Larach MG., Kunselman AR. The sensitivity and specificity of the caffeine-halothane contracture test: a report from the North American Malignant Hyperthermia Registry. The North American Malignant Hyperthermia Registry of MHAUS. Anesthesiology. 1998. 88:579–88.
4.Ording H., Brancadoro V., Cozzolino S., Ellis FR., Glauber V., Gonano EF, et al. In vitro contracture test for diagnosis of malignant hyperthermia following the protocol of the European MH Group: results of testing patients surviving fulminant MH and unrelated low-risk subjects. The European Malignant Hyperthermia Group. Acta Anaesthesiol Scand. 1997. 41:955–66.
5.MacLennan DH., Duff C., Zorzato F., Fujii J., Phillips M., Korneluk RG, et al. Ryanodine receptor gene is a candidate for predisposition to malignant hyperthermia. Nature. 1990. 343:559–61.

6.Schwemmle S., Wolff K., Palmucci LM., Grimm T., Lehmann-Horn F., Hubner C, et al. Multipoint mapping of the central core disease locus. Genomics. 1993. 17:205–7.

7.Quane KA., Healy JM., Keating KE., Manning BM., Couch FJ., Palmucci LM, et al. Mutations in the ryanodine receptor gene in central core disease and malignant hyperthermia. Nat Genet. 1993. 5:51–5.

8.Gillard EF., Otsu K., Fujii J., Duff C., de Leon S., Khanna VK, et al. Polymorphisms and deduced amino acid substitutions in the coding sequence of the ryanodine receptor (RYR1) gene in individuals with malignant hyperthermia. Genomics. 1992. 13:1247–54.
9.Zhang Y., Chen HS., Khanna VK., De Leon S., Phillips MS., Schappert K, et al. A mutation in the human ryanodine receptor gene associated with central core disease. Nat Genet. 1993. 5:46–50.

10.Levitt RC., Olckers A., Meyers S., Fletcher JE., Rosenberg H., Isaacs H, et al. Evidence for the localization of a malignant hyperthermia susceptibility locus (MHS2) to human chromosome 17q. Genomics. 1992. 14:562–6.

11.Iles DE., Lehmann-Horn F., Scherer SW., Tsui LC., Olde Weghuis D., Suijkerbuijk RF, et al. Localization of the gene encoding the alpha 2/delta-subunits of the L-type voltage-dependent calcium channel to chromosome 7q and analysis of the segregation of flanking markers in malignant hyperthermia susceptible families. Hum Mol Genet. 1994. 3:969–75.
12.Sudbrak R., Procaccio V., Klausnitzer M., Curran JL., Monsieurs K., van Broeckhoven C, et al. Mapping of a further malignant hyperthermia susceptibility locus to chromosome 3q13.1. Am J Hum Genet. 1995. 56:684–91.
13.Gregg RG., Couch F., Hogan K., Powers PA. Assignment of the human gene for the alpha 1 subunit of the skeletal muscle DHP-sensitive Ca2+ channel (CACNL1A3) to chromosome 1q31-q32. Genomics. 1993. 15:107–12.
14.Robinson RL., Curran JL., Ellis FR., Halsall PJ., Hall WJ., Hopkins PM, et al. Multiple interacting gene products may influence susceptibility to malignant hyperthermia. Ann Hum Genet. 2000. 64:307–20.

15.Robinson RL., Monnier N., Wolz W., Jung M., Reis A., Nuernberg G, et al. A genome wide search for susceptibility loci in three European malignant hyperthermia pedigrees. Hum Mol Genet. 1997. 6:953–61.

16.Brini M. Ryanodine receptor defects in muscle genetic diseases. Biochem Biophys Res Commun. 2004. 322:1245–55.

17.Benkusky NA., Farrell EF., Valdivia HH. Ryanodine receptor channelopathies. Biochem Biophys Res Commun. 2004. 322:1280–5.

19.Ogawa Y., Kurebayashi N., Murayama T. Ryanodine receptor iso-forms in excitation-contraction coupling. Adv Biophys. 1999. 36:27–64.

20.Phillips MS., Fujii J., Khanna VK., DeLeon S., Yokobata K., de Jong PJ, et al. The structural organization of the human skeletal muscle ryanodine receptor (RYR1) gene. Genomics. 1996. 34:24–41.
21.McCarthy TV., Quane KA., Lynch PJ. Ryanodine receptor mutations in malignant hyperthermia and central core disease. Hum Mutat. 2000. 15:410–7.

22.Ibarra M CA., Wu S., Murayama K., Minami N., Ichihara Y., Kikuchi H, et al. Malignant hyperthermia in Japan: mutation screening of the entire ryanodine receptor type 1 gene coding region by direct sequencing. Anesthesiology. 2006. 104:1146–54.
24.Urwyler A., Deufel T., McCarthy T., West S. Guidelines for molecular genetic detection of susceptibility to malignant hyperthermia. Br J Anaesth. 2001. 86:283–7.

25.Sei Y., Sambuughin N., Muldoon S. Malignant hyperthermia genetic testing in North America Working Group Meeting. Bethesda, Maryland. September 4-5, 2002. Anesthesiology. 2004. 100:464–5.
26.Kim DC., Kim DS. Identification of G7304A mutation in the ryanodine receptor type 1 gene in a patient with malignant hyperthermia and an extended pedigree study in a Korean malignant hyperthermia family. Korean J Anesthesiol. 2003. 44:56–64. (김동찬및김달식. 한국인 악성고열증 환자에서 발견된 Ryanodine 수용체(RYR1) G-7304A (Arg2435His) 유전자변이와가계구성원의유전자변이추적조사. 대한마취과학회지 2003;44: 56-64.).

27.Sambuughin N., Sei Y., Gallagher KL., Wyre HW., Madsen D., Nelson TE, et al. North American malignant hyperthermia population: screening of the ryanodine receptor gene and identification of novel mutations. Anesthesiology. 2001. 95:594–9.
28.Sambuughin N., Holley H., Muldoon S., Brandom BW., de Bantel AM., Tobin JR, et al. Screening of the entire ryanodine receptor type 1 coding region for sequence variants associated with malignant hyperthermia susceptibility in the north american population. Anesthesiology. 2005. 102:515–21.

29.Kraev N., Loke JC., Kraev A., MacLennan DH. Protocol for the sequence analysis of ryanodine receptor subtype 1 gene transcripts from human leukocytes. Anesthesiology. 2003. 99:289–96.

30.Treves S., Anderson AA., Ducreux S., Divet A., Bleunven C., Grasso C, et al. Ryanodine receptor 1 mutations, dysregulation of calcium homeostasis and neuromuscular disorders. Neuromuscul Disord. 2005. 15:577–87.

31.Guis S., Figarella-Branger D., Monnier N., Bendahan D., Kozak-Ribbens G., Mattei JP, et al. Multiminicore disease in a family susceptible to malignant hyperthermia: histology, in vitro contracture tests, and genetic characterization. Arch Neurol. 2004. 61:106–13.
32.Manning BM., Quane KA., Ording H., Urwyler A., Tegazzin V., Lehane M, et al. Identification of novel mutations in the ryanodine-receptor gene (RYR1) in malignant hyperthermia: genotype-phenotype correlation. Am J Hum Genet. 1998. 62:599–609.
33.Oyamada H., Oguchi K., Saitoh N., Yamazawa T., Hirose K., Kawana Y, et al. Novel mutations in C-terminal channel region of the ryanodine receptor in malignant hyperthermia patients. Jpn J Pharmacol. 2002. 88:159–66.

34.Tong J., Oyamada H., Demaurex N., Grinstein S., McCarthy TV., MacLennan DH. Caffeine and halothane sensitivity of intracellular Ca2+ release is altered by 15 calcium release channel (ryanodine receptor) mutations associated with malignant hyperthermia and/or central core disease. J Biol Chem. 1997. 272:26332–9.

Fig. 1.
Haplotype analysis and segregation of the RYR1 mutations and variations p.Arg2458His (in family 1), p.Arg2508Cys (in family 2), p.Met4022Val and p.Arg2676Trp (in family 3), p.Glu2669Lys (in family 4), and p.Arg2435His and p.Ala4295Val (in family 5). The arrow denotes the proband who experienced malignant hyperthermia crises. Black shapes indicate individuals with a past history of malignant hyperthermia attack, and white shapes with a question mark denote individuals for whom disease status is unknown. Segregation of the respective mutations and variations is also indicated: a plus sign (+) indicates that an individual is heterozygous for the mutant allele, and a minus sign (-) indicates that an individual is homozygous for the normal allele. The results of marker typing and mutation identification are shown in order: D19S191-D19S220-RYR1 mutations or variations-D19S422-D19S190-D19S223.
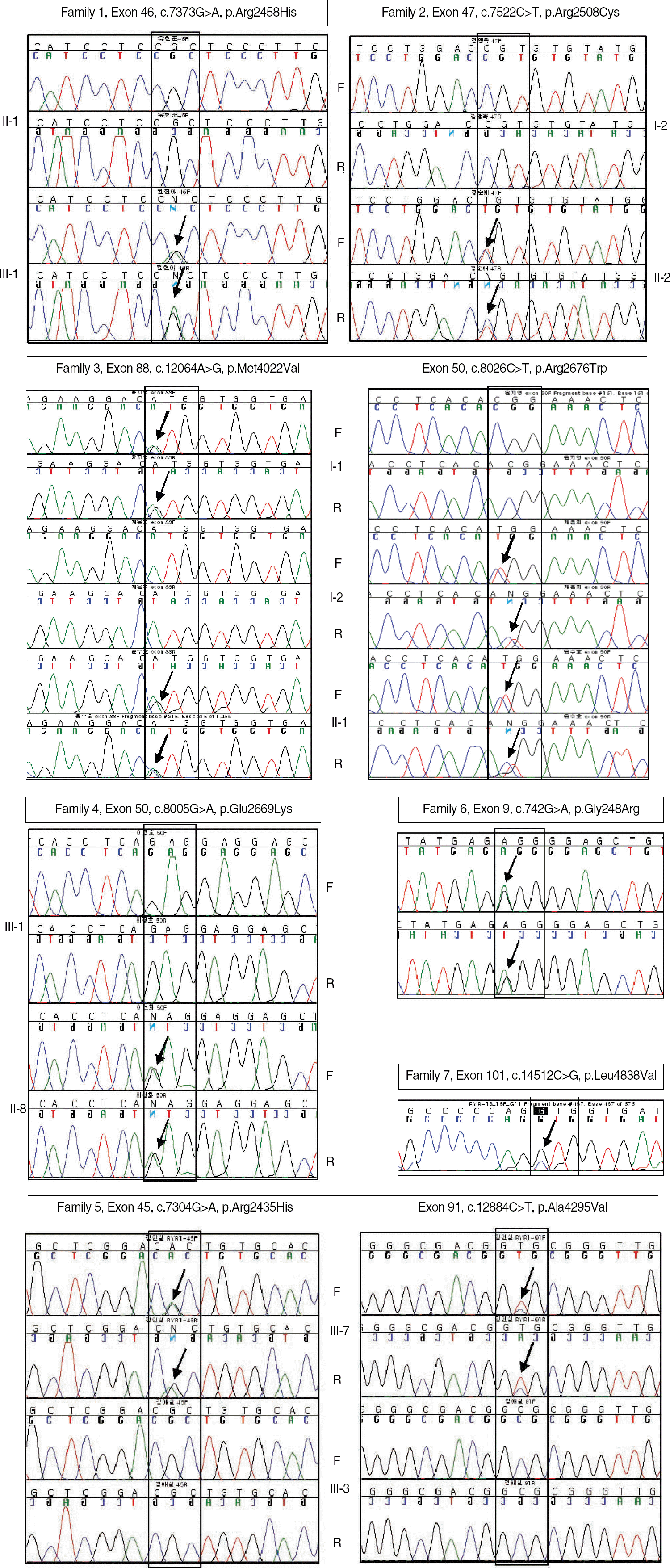
Fig. 2.
DNA sequencing of the entire coding region of the RYR1 gene, in which c.7373CGC>CAC∗ in exon 46 (from family 1); c.7522CGT> TGT in exon 47 (from family 2); c.12064ATG>GTG and c.8026CGG>TGG in exon 88 and 50, respectively (from family 3); c.8005GAG> AAG in exon 50 (from family 4); c.7304CGC>CAC and c.12884GCG>GTG in exon 45 and 91, respectively (from family 5); c.742GGG> AGG in exon 9 (from family 6); and c.14512CTG>GTG in exon 101 (from family 7) were detected. Individuals without a mutation or variation showed a single peak, whereas individuals with mutations or variations showed two superimposed peaks (arrows), indicating a heterozygous missense mutation or variation. ∗Residue numbering within the human RYR1 cDNA (accession number: NM_000540). Abbreviations: F, forward sequencing; R, reverse sequencing. DNA sequencing of the entire coding region of the RYR1 gene, in which c.7373CGC>CAC∗ in exon 46 (from family 1); c.7522CGT> TGT in exon 47 (from family 2); c.12064ATG>GTG and c.8026CGG>TGG in exon 88 and 50, respectively (from family 3); c.8005GAG> AAG in exon 50 (from family 4); c.7304CGC>CAC and c.12884GCG>GTG in exon 45 and 91, respectively (from family 5); c.742GGG> AGG in exon 9 (from family 6); and c.14512CTG>GTG in exon 101 (from family 7) were detected. Individuals without a mutation or variation showed a single peak, whereas individuals with mutations or variations showed two superimposed peaks (arrows), indicating a heterozygous missense mutation or variation. ∗Residue numbering within the human RYR1 cDNA (accession number: NM_000540).
Abbreviations: F, forward sequencing; R, reverse sequencing.




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download