Abstract
Background:
Molecular methods have enabled rapid diagnosis of aseptic meningitis and have reduced both unnecessary therapeutic interventions and medical costs. In this study, we evaluated the analytical performance of the recently developed Real-Q Enterovirus Quantification kit (BioSewoom Inc., Korea).
Methods:
We evaluated the detection limit, precision, linearity, and cross-reactivity of the Real-Q Enterovirus Quantification kit and compared it with the conventional PCR method. From March to September 2009, we tested 91 CSF specimens from patients who visited the pediatrics department of the university hospital with symptoms of aseptic meningitis or infantile sepsis, and we also tested 48 CSF specimens from patients with febrile convulsion for differential diagnosis.
Results:
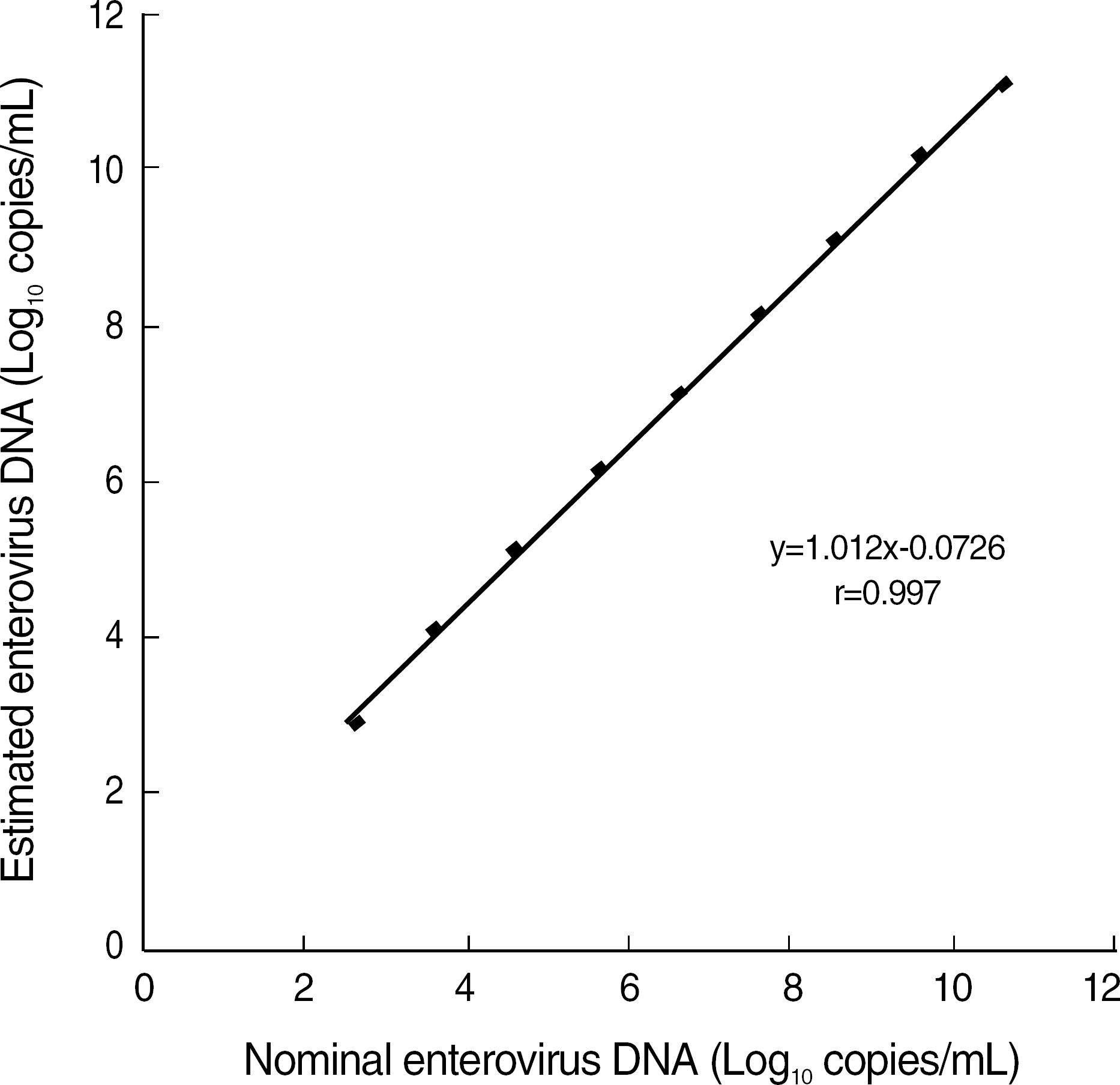
The Real-Q Enterovirus Quantification kit showed good linearity (r=0.997) within a range from 3×102 to 3×1010 copies/mL, and the detection limit of the kit was 83 copies/mL. The within-run, between-run, and between-day CVs were 5.3-7.6%, 9.5-12.3%, and 11.4-13.4%, respectively. There was no cross reactivity between enteroviruses and various microorganisms. Positive results were obtained for 39.1% (25/64) of the patients suspected of aseptic meningitis and 44.4% (12/27) of the patients suspected of infantile sepsis. However, among the 48 children with febrile conversion, only 4 were positive for enterovirus. Further, the concordance with conventional PCR was high (73/74).
Conclusions:
The Real-Q Enterovirus Quantification kit showed excellent linearity and high reliability with a broad reportable range. It showed good detection rate when used with clinical specimens and also showed a high concordance with the conventional method. Therefore, this assay would be clinically useful not only in diagnosis of aseptic meningitis but also in differential diagnosis of infantile sepsis.
Go to : 
REFERENCES
1.Pallansch MA., Roos R. Enteroviruses, polioviruses, coxsackieviruses, echoviruses, and newer enteroviruses. Knipe DM, Howley PM, editors. Fields virology. 5th ed.Philadelphia: Lippincott Williams & Wilkins;2007. p. 839–93.
2.Stanway G., Brown F., Christian P. Picornaviridae. Fauquet CM, Mayo MA, editors. Virus taxonomy: classification and nomenclature of viruses: 8th report of the International Committee on the Taxonomy of Viruses. Amsterdam: Elsevier Academic Press;2005. p. 757–78.
3.Nix WA., Oberste MS., Pallansch MA. Sensitive, seminested PCR amplification of VP1 sequences for direct identification of all enterovirus serotypes from original clinical specimens. J Clin Microbiol. 2006. 44:2698–704.

4.Khetsuriani N., Lamonte-Fowlkes A., Oberst S., Pallansch MA. Enterovirus surveillance—United States, 1970-2005. MMWR Surveill Summ. 2006. 55:1–20.
5.Sawyer MH., Holland D., Aintablian N., Connor JD., Keyser EF., Waecker NJ Jr. Diagnosis of enteroviral central nervous system infection by polymerase chain reaction during a large community outbreak. Pediatr Infect Dis J. 1994. 13:177–82.

6.Ramers C., Billman G., Hartin M., Ho S., Sawyer MH. Impact of a diagnostic cerebrospinal fluid enterovirus polymerase chain reaction test on patient management. JAMA. 2000. 283:2680–5.

7.Verstrepen WA., Kuhn S., Kockx MM., Van De Vyvere ME., Mertens AH. Rapid detection of enterovirus RNA in cerebrospinal fluid specimens with a novel single-tube real-time reverse transcription-PCR assay. J Clin Microbiol. 2001. 39:4093–6.

8.Heo SR., Jin SK., Chang HE., Park KU., Song J., Kim EC. Detection of enterovirus in cerebrospinal fluid by real-time nested reverse transcription polymerase chain reaction. Korean J Lab Med. 2006. 26:9–13. (허세란, 진선경, 장호은, 박경운, 송정한, 김의종. 뇌척수액에서 실시간-이중-역전사중합효소연쇄반응을 이용한 장바이러스의 검출. 대한진단검사의학회지 2006;26:9-13.).

9.Pillet S., Billaud G., Omar S., Lina B., Pozzetto B., Schuffenecker I. Multicenter evaluation of the ENTEROVIRUS R-gene real-time RT-PCR assay for the detection of enteroviruses in clinical specimens. J Clin Virol. 2010. 47:54–9.

10.National Committee for Clinical Laboratory Standards. Protocols for determination of limits of detection and limits of quantitation: approved guideline (EP17-A). 2nd ed.Wayne, PA: NCCLS;2004.
11.Noordhoek GT., Weel JF., Poelstra E., Hooghiemstra M., Brandenburg AH. Clinical validation of a new real-time PCR assay for detection of enteroviruses and parechoviruses, and implications for diagnostic procedures. J Clin Virol. 2008. 41:75–80.

12.National Committee for Clinical Laboratory Standards. Evaluation of the precision performance of clinical chemistry devices: approved guideline (EP5-A2). 2nd ed.Wayne, PA: NCCLS;2004.
13.National Committee for Clinical Laboratory Standards. Evaluation of the linearity of quantitative measurement prodedures: a statistical approach: approved guideline (EP6-A). 2nd ed.Wayne, PA: NCCLS;2003.
14.Rotbart HA. Nucleic acid detection systems for enteroviruses. Clin Microbiol Rev. 1991. 4:156–68.

15.Archimbaud C., Chambon M., Bailly JL., Petit I., Henquell C., Mirand A, et al. Impact of rapid enterovirus molecular diagnosis on the management of infants, children, and adults with aseptic meningitis. J Med Virol. 2009. 81:42–8.

16.Kammerer U., Kunkel B., Korn K. Nested PCR for specific detection and rapid identification of human picornaviruses. J Clin Microbiol. 1994. 32:285–91.

17.Heim A., Schumann J. Development and evaluation of a nucleic acid sequence based amplification (NASBA) protocol for the detection of enterovirus RNA in cerebrospinal fluid samples. J Virol Methods. 2002. 103:101–7.

18.Petitjean J., Vabret A., Dina J., Gouarin S., Freymuth F. Development and evaluation of a real-time RT-PCR assay on the LightCycler for the rapid detection of enterovirus in cerebrospinal fluid specimens. J Clin Virol. 2006. 35:278–84.

19.Monpoeho S., Coste-Burel M., Costa-Mattioli M., Besse B., Chomel JJ., Billaudel S, et al. Application of a real-time polymerase chain reaction with internal positive control for detection and quantification of enterovirus in cerebrospinal fluid. Eur J Clin Microbiol Infect Dis. 2002. 21:532–6.
Go to : 
Table 1.
The list of viruses and bacteria used for evaluating cross-reactions of Real-Q Enterovirus Quantification kit




 PDF
PDF ePub
ePub Citation
Citation Print
Print


 XML Download
XML Download